Preparation method for (R)-3-chloro-1,2-propanediol
A technology of propylene glycol and propylene oxide, applied in the preparation of organic compounds, hydrolysis preparation, chemical instruments and methods, etc., can solve the problems of less than 90%, multiple impurity peaks, affecting product economic benefits and market competition, etc. Achieve low moisture content, excellent yield, excellent overall purity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0016] The present invention provides a preparation method of (R)-3-chloro-1,2-propanediol, the preparation method comprising:
[0017] 1) In the presence of an acid catalyst, (S)-3-chloro-1,2-propylene oxide and water are subjected to a ring-opening reaction to obtain a ring-opening mixture;
[0018] 2) Neutralizing the lye and the ring-opening mixture to obtain a neutralized mixture;
[0019] 3) Remove the water in the neutralized mixture, and then filter to obtain the filtrate;
[0020] 4) Fractional distillation of the filtrate at 30-35 Pa and 125-128°C to prepare (R)-3-chloro-1,2-propanediol.
[0021] In the present invention, the acidic catalyst can be any conventional acidic catalyst in the art, and the specific type can be selected in a wide range, but in order to make the prepared (R)-3-chloro-1,2-propanediol more Excellent overall purity (especially chiral purity), yield and low water content. Preferably, in step 1), the acidic catalyst is selected from one or more of sulfuri...
Embodiment 1
[0030] 1) At 108°C, 100 g of (S)-3-chloro-1,2-propylene oxide, 60 g of water and 1.5 g of sulfuric acid are subjected to a ring-opening reaction for 6 hours to obtain a ring-opening mixture;
[0031] 2) At 25° C., 15 g of sodium hydroxide aqueous solution (the mass fraction of sodium hydroxide is 5%) and 100 g of the ring-opening mixture are neutralized for 15 minutes to obtain a neutralized mixture (pH 7.0);
[0032] 3) Remove water from the neutralized mixture by distillation under reduced pressure (the water content of the mixture after water removal is 0.3%), and then filter to obtain a filtrate;
[0033] 4) Fractional distillation of the filtrate at 33 Pa and 126°C to prepare (R)-3-chloro-1,2-propanediol.
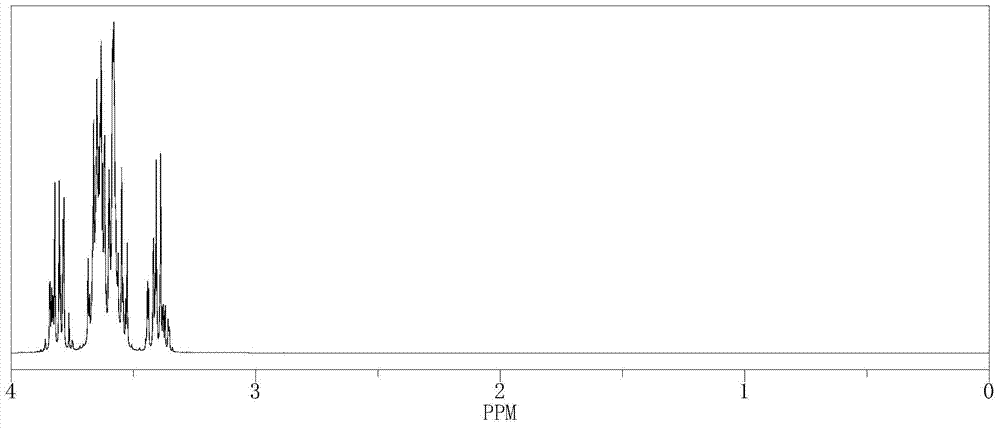
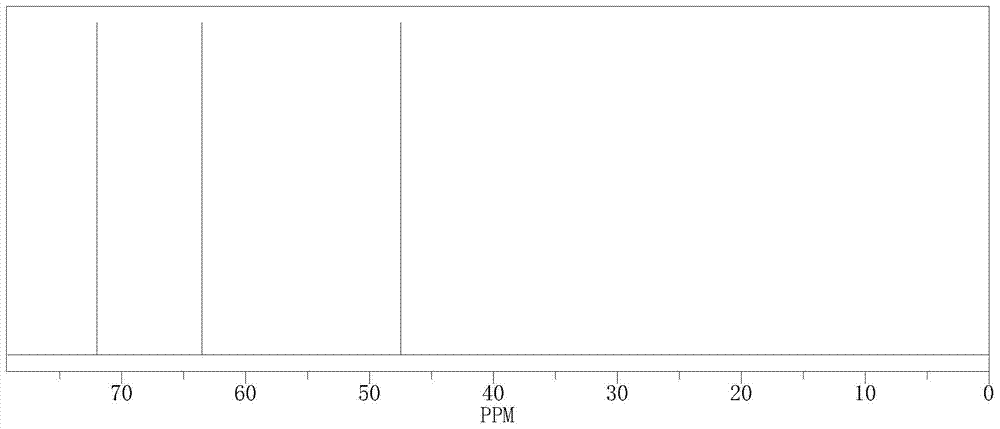
[0034] The hydrogen nuclear magnetic spectrum of (R)-3-chloro-1,2-propanediol see figure 1 , See figure 2 .
[0035] See Table 1 for the yield, total purity, chiral purity and water content parameters of (R)-3-chloro-1,2-propanediol in this example.
Embodiment 2
[0037] (R)-3-chloro-1,2-propanediol was prepared according to the method of Example 1, except that in step 3), the water content of the mixture after water removal was 0.1%.
[0038] The data of the hydrogen nuclear magnetic spectrum and the carbon nuclear magnetic spectrum of the (R)-3-chloro-1,2-propanediol are consistent with the characterization map in Example 1.
[0039] See Table 1 for the yield, total purity, chiral purity and water content parameters of (R)-3-chloro-1,2-propanediol in this example.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com