Lansoprazole or dextral lansoprazole crystal type compound and preparation method thereof
A technology of dexlansoprazole trihydrate and racemic lansoprazole trihydrate is applied in the field of medicine, which can solve problems such as unsatisfactory results and influence on drug quality, achieve small content changes, improve dissolution rate, The effect of good fluidity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0049] [embodiment 1] the preparation of lansoprazole crystal form compound
[0050] (1) Get lansoprazole crude product 100g, add mixed solution A2000ml at 45 DEG C, stir until all dissolvings, get lansoprazole solution, wherein said mixed solution A is N-methylpyrrolidone and methyl alcohol with 4: Prepared with a volume ratio of 1;
[0051] (2) The lansoprazole solution obtained in step 1) is naturally cooled to room temperature, and the mixed solution B is added under stirring at a stirring speed of 1480r / min. After the addition is completed, the temperature is lowered to 2° C., and crystals are precipitated, wherein the The volume ratio of mixed solution B and lansoprazole solution is 20:1, and described mixed solution B is that acetone and water are prepared with the volume ratio of 1:3.5;
[0052] (3) Stand still in an environment with a temperature of 2° C. for 3 hours, filter, wash the filter cake with methanol, and dry in vacuum to obtain the lansoprazole crystal com...
Embodiment 2-8
[0059]
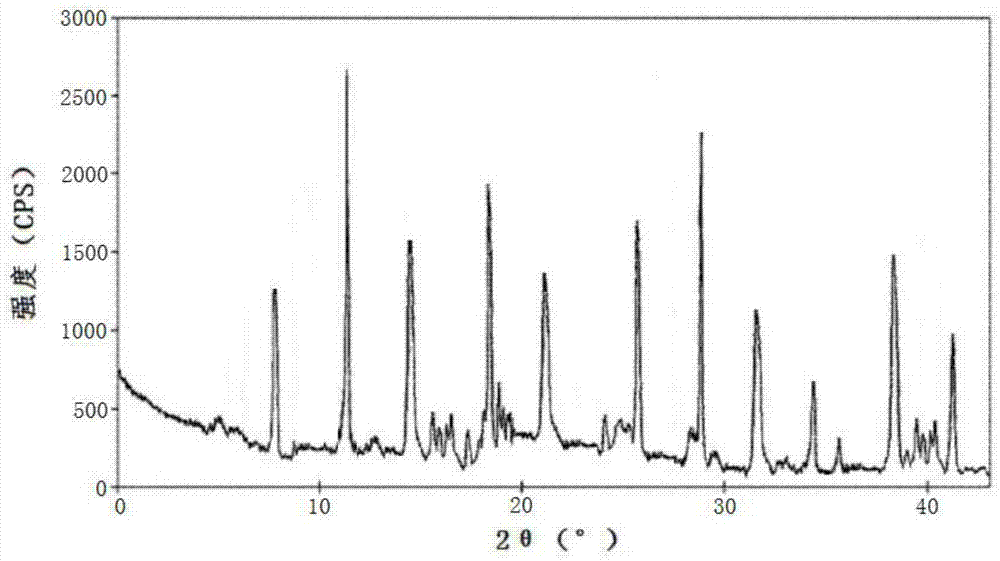
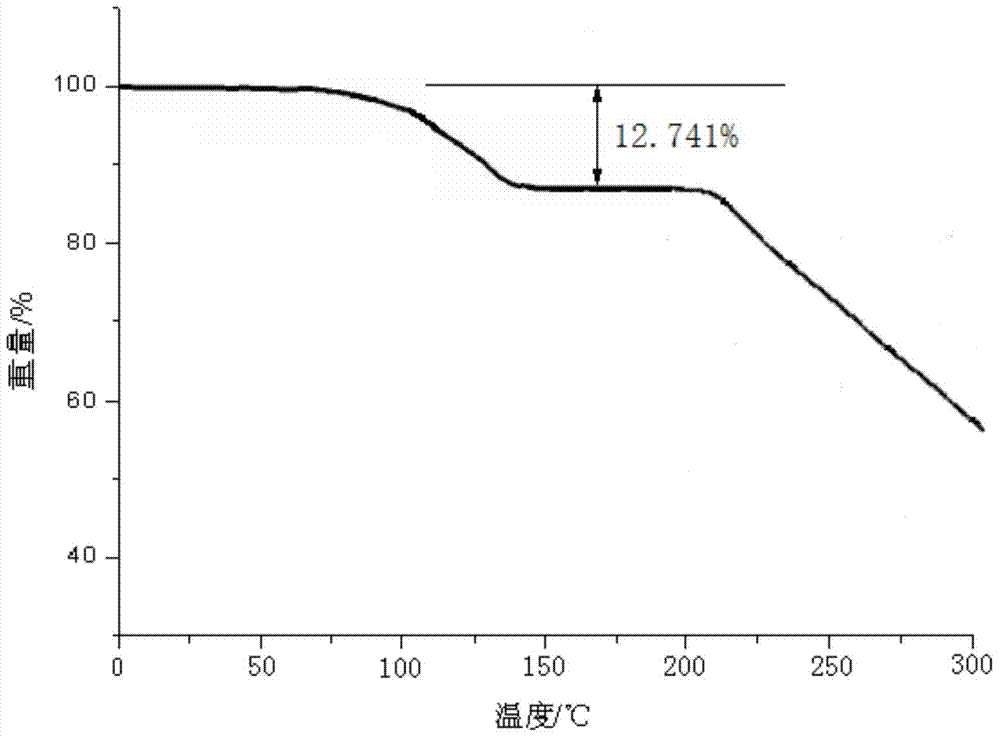
[0060] The lansoprazole crystal form compound that embodiment 2-8 makes adopts U.S. Perkin-Elmer company PE 2400 II elemental analyzer, elemental analysis (%) measured value is close to embodiment 1; Use Cu-Kα ray measurement to obtain The X-ray powder diffraction spectrogram is similar to Example 1; Cassette type moisture determination is similar to Example 1;
Embodiment 9
[0061] [embodiment 9] preparation of dexlansoprazole crystal form compound
[0062] (1) Get dexlansoprazole crude product 100g, add mixed solution A2000ml at 45 ℃, stir until all dissolving, get dexlansoprazole solution, wherein said mixed solution A is N-methylpyrrolidone and Methanol is prepared at a volume ratio of 4:1;
[0063] (2) The dexlansoprazole solution obtained in step 1) is naturally cooled to room temperature, and the mixed solution B is added under stirring at a stirring speed of 1480r / min. The volume ratio of described mixed solution B and dexlansoprazole solution is 20:1, and described mixed solution B is that acetone and water are prepared with the volume ratio of 1:3.5;
[0064] (3) Stand still in an environment with a temperature of 2°C for 3 hours, filter, wash the filter cake with methanol, and dry in vacuo to obtain the crystalline compound of D-lansoprazole with an optical purity of 99.9%.
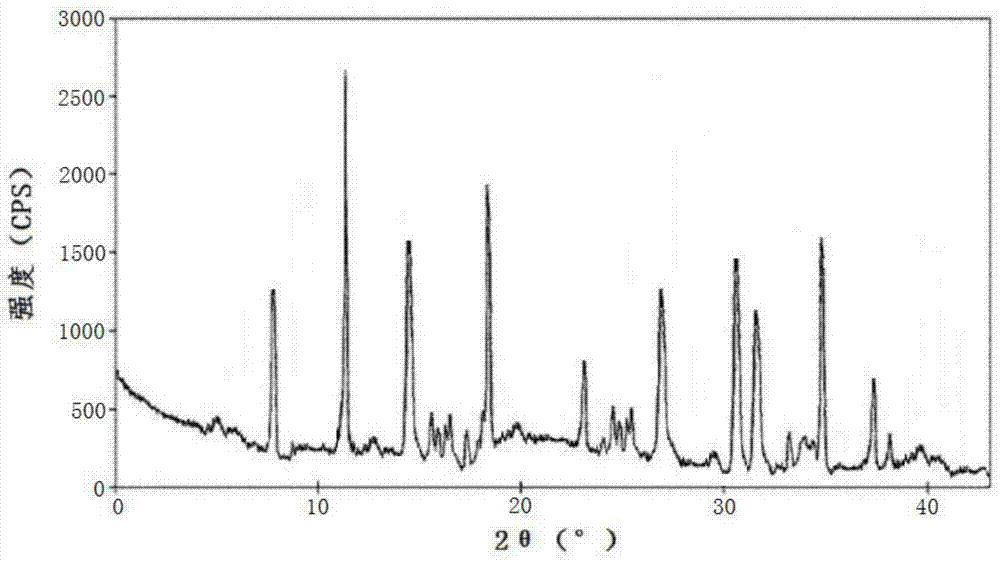
[0065] Using the PE 2400 II elemental analyzer of Perkin-Elm...
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle diameter | aaaaa | aaaaa |
| optical purity | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com