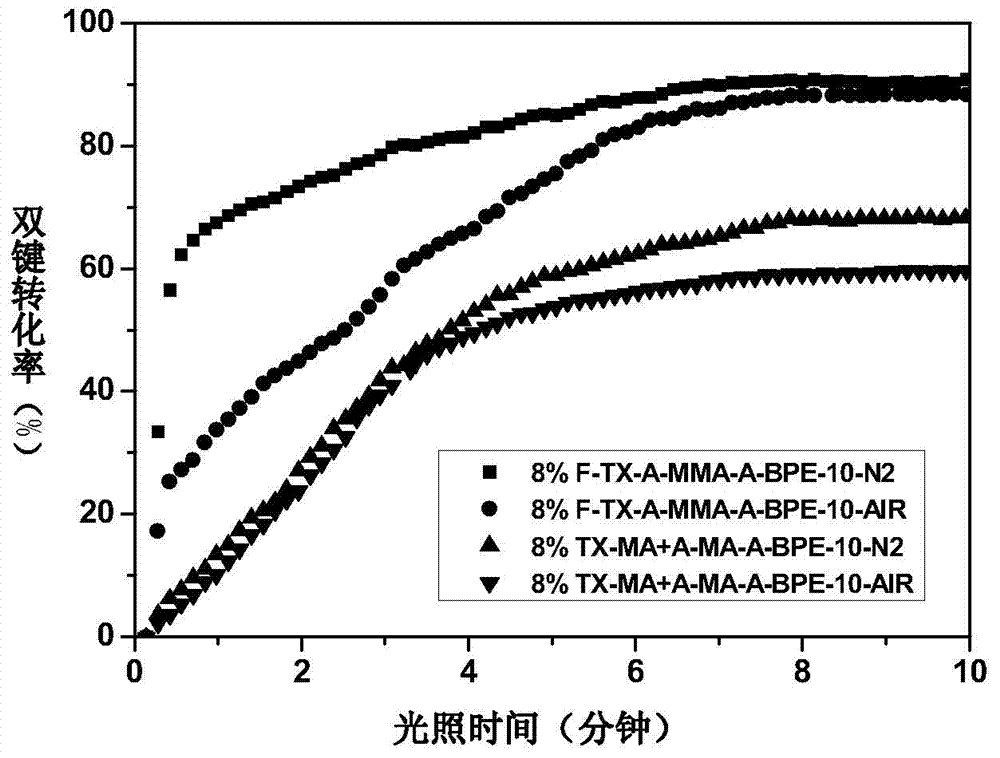
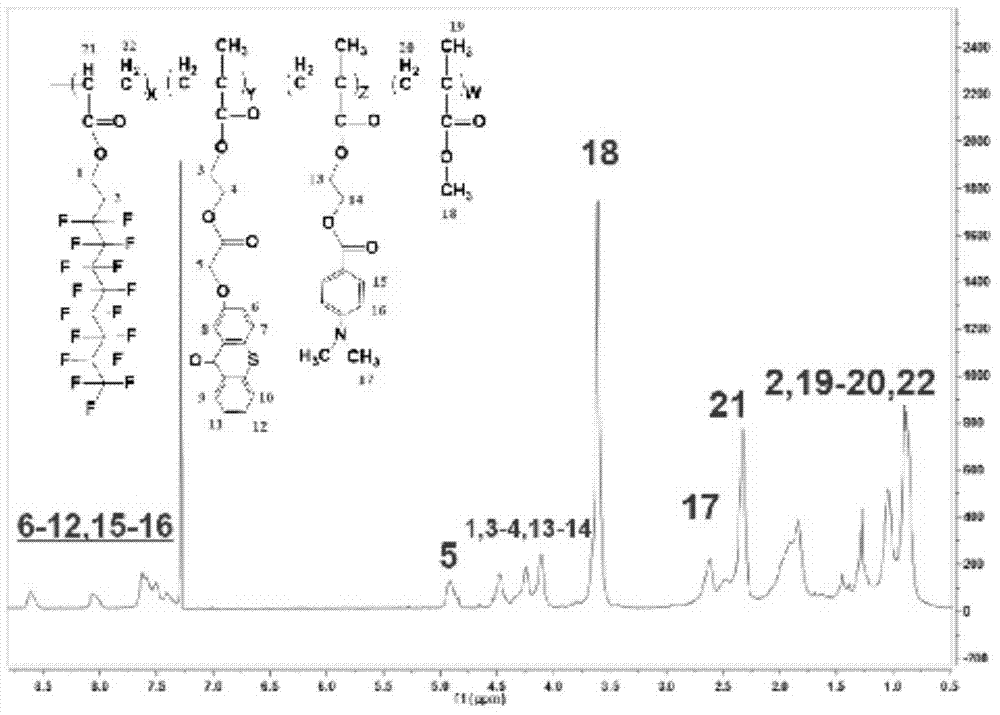
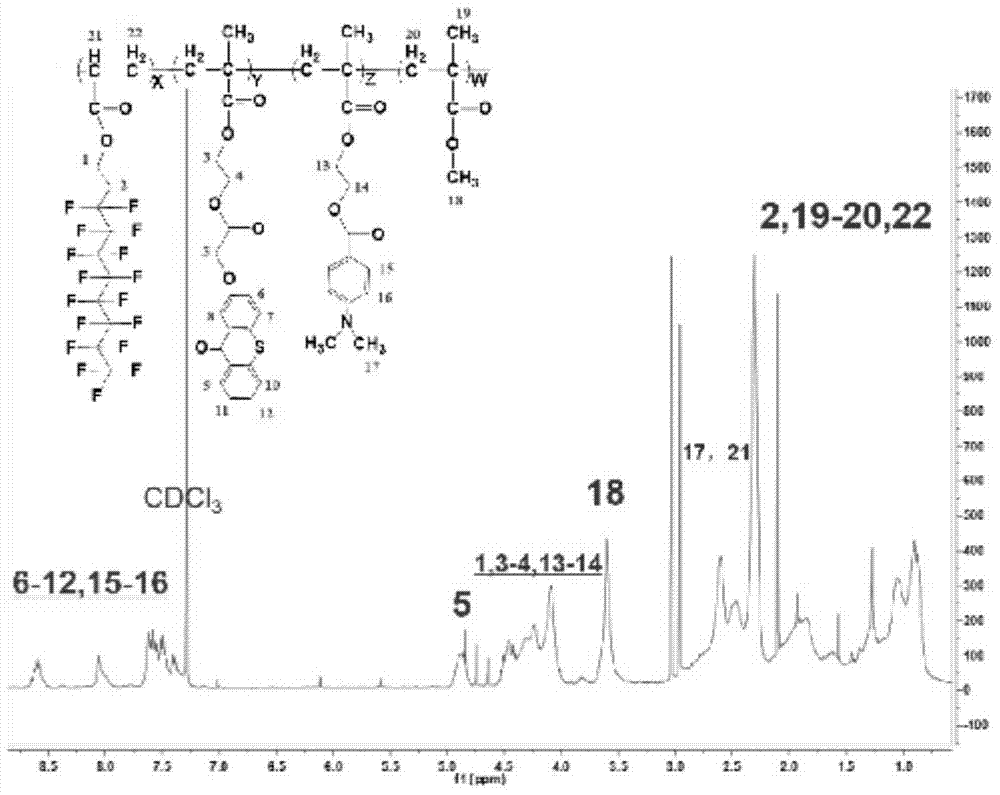
Fluorated hydrogen abstracting type macromolecular photoinitiator, and preparation method and application thereof
A photoinitiator and polymer technology, applied in the field of fluorinated hydrogen abstraction polymer photoinitiator, can solve the problems of residue, yellowing, easy migration, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0039] A fluorinated hydrogen abstraction polymer photoinitiator P (F-TX-A-MMA), the structural formula is as follows:
[0040]
[0041] The preparation method is as follows:
[0042] (1) 1 mole of O-(thioxanthone-[2]-yl)-oxyacetic acid and 1.2 moles of 2-hydroxyethyl methacrylate are dissolved in dichloromethane, ice-water bath, and then add 0.1 mole 4-dimethylaminopyridine and 1 mole of 1-(3-dimethylaminopropyl)-3-ethylcarbodiimide hydrochloride were used as catalyst and dehydrating agent respectively, stirred, and reacted for 24 hours at normal temperature, and used Precipitate with a large amount of deionized water, filter with suction, and dry in a constant temperature vacuum oven at 70°C to prepare acrylate containing photoinitiator thioxanthone;
[0043] (2) 1 mole of p-dimethylaminobenzoic acid and 1.2 moles of 2-hydroxyethyl methacrylate are dissolved in dichloromethane, then add 0.1 mole of 4-dimethylaminopyridine and 1 mole of 1-( 3-Dimethylaminopropyl)-3-ethyl...
Embodiment 2
[0048] A fluorinated hydrogen abstraction polymer photoinitiator P(F-BP-DEMA-S), the structural formula is as follows:
[0049]
[0050] The preparation method is as follows:
[0051] (1) 1.2 moles of 4-chloromethylstyrene and 1 mole of hydroxybenzophenone are dissolved in acetonitrile, 1 mole of anhydrous potassium carbonate and catalyst amount of potassium iodide are added, refluxed for 24 hours, deionized water precipitation, Filter with suction and dry in a constant temperature vacuum oven at 60°C to obtain 3-styrene methyl ether-benzophenone.
[0052] (2) 3-Styrene methyl ether-benzophenone, perfluorooctyl ethyl acrylate, N,N-dimethylaminoethyl methacrylate and styrene in azo The copolymerization reaction of diisobutyl nitrile occurs under thermal initiation at 60°C, reacts for 24 hours, precipitates in ether, and dries in a constant temperature vacuum oven at 40°C to obtain the target product.
[0053] Figure 4 It is the structural diagram of P(F-BP-DEMA-S) synthe...
Embodiment 3
[0055] A fluorinated hydrogen abstraction polymer photoinitiator P (F-TX-DEMA-MMA), the structural formula is as follows:
[0056]
[0057] The preparation method is as follows:
[0058] (1) 1 mole of O-(thioxanthone-[2]-yl)-oxyacetic acid and 1.2 moles of 2-hydroxyethyl methacrylate are dissolved in dichloromethane, ice-water bath, and then add 0.1 mole 4-dimethylaminopyridine and 1 mole of 1-(3-dimethylaminopropyl)-3-ethylcarbodiimide hydrochloride as catalyst and dehydrating agent, stirred, and reacted for 24 hours at normal temperature, using a large amount of Precipitate with deionized water, filter with suction, and dry in a constant temperature vacuum oven at 70°C to prepare acrylate containing photoinitiator thioxanthone;
[0059] (2) According to a certain feeding ratio (X:Y:Z:W=2:30:10:10), the photoinitiator-containing thioxanthone acrylate, the co-initiator-containing amine acrylate (methacrylic acid- N,N-dimethylaminoethyl ester), methyl methacrylate and perf...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com