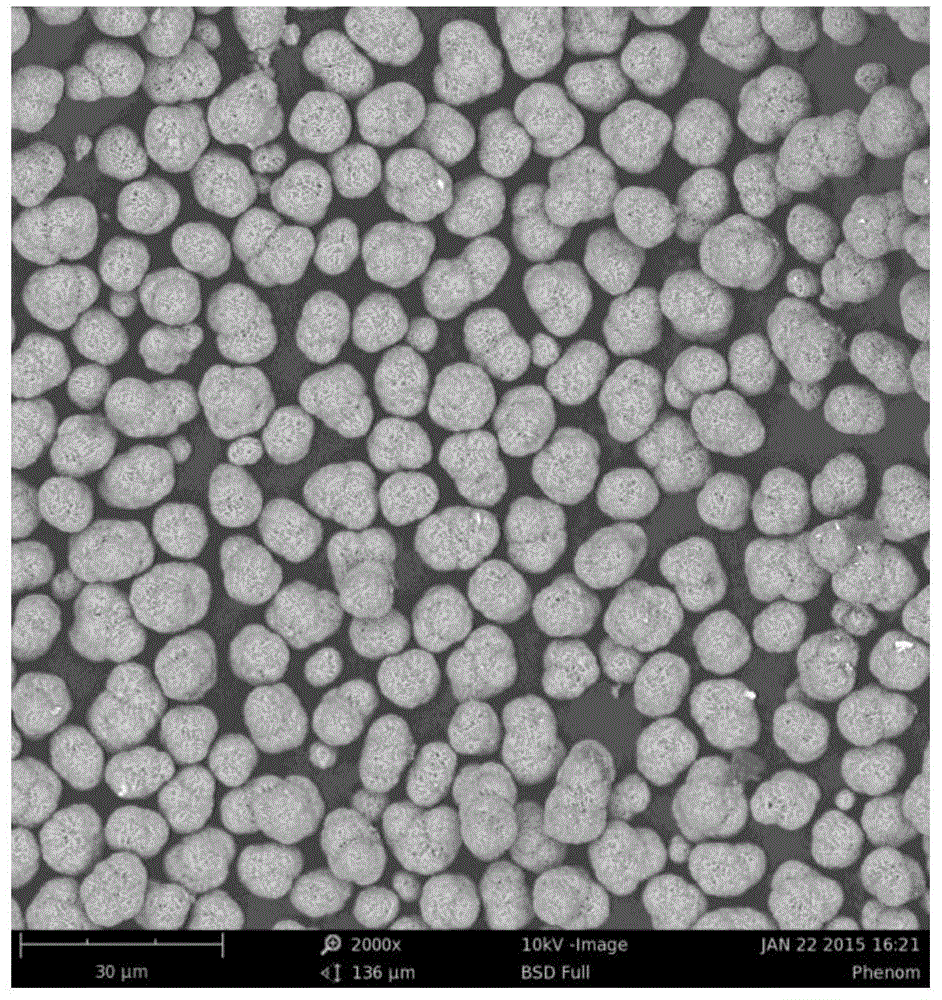
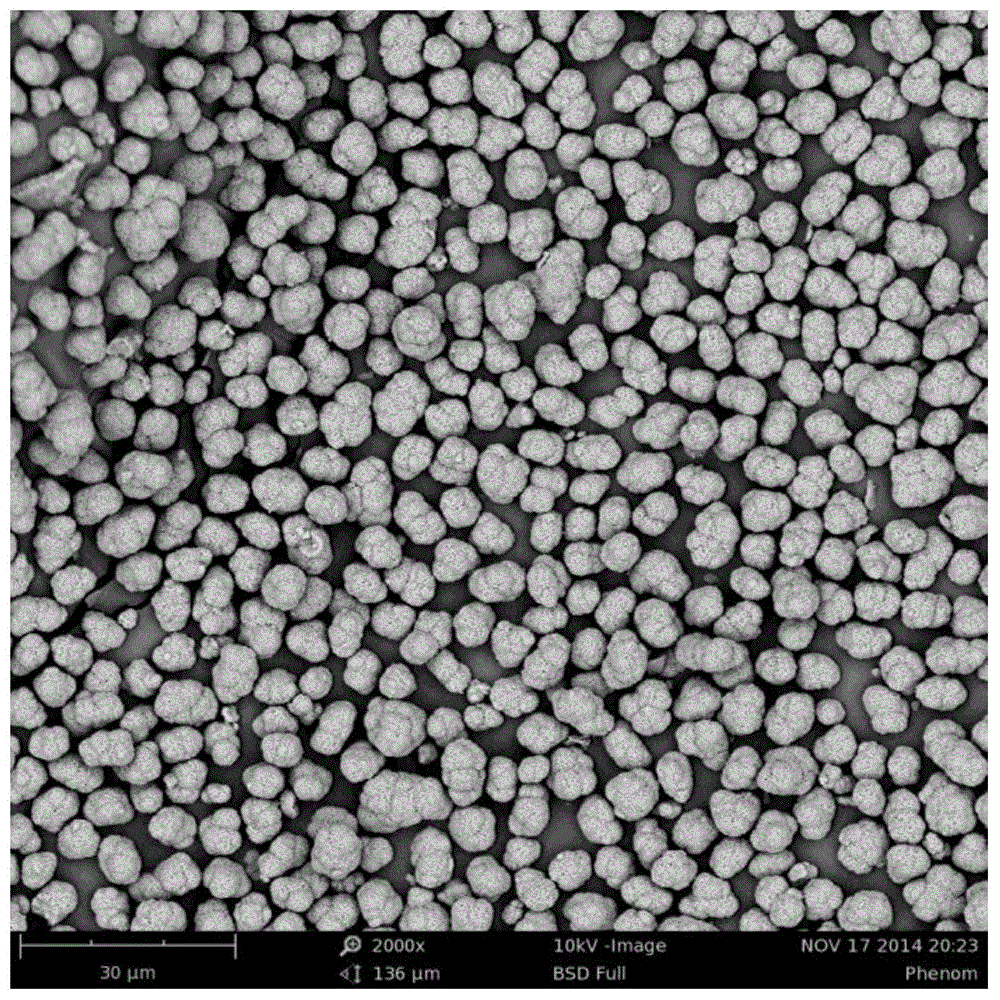
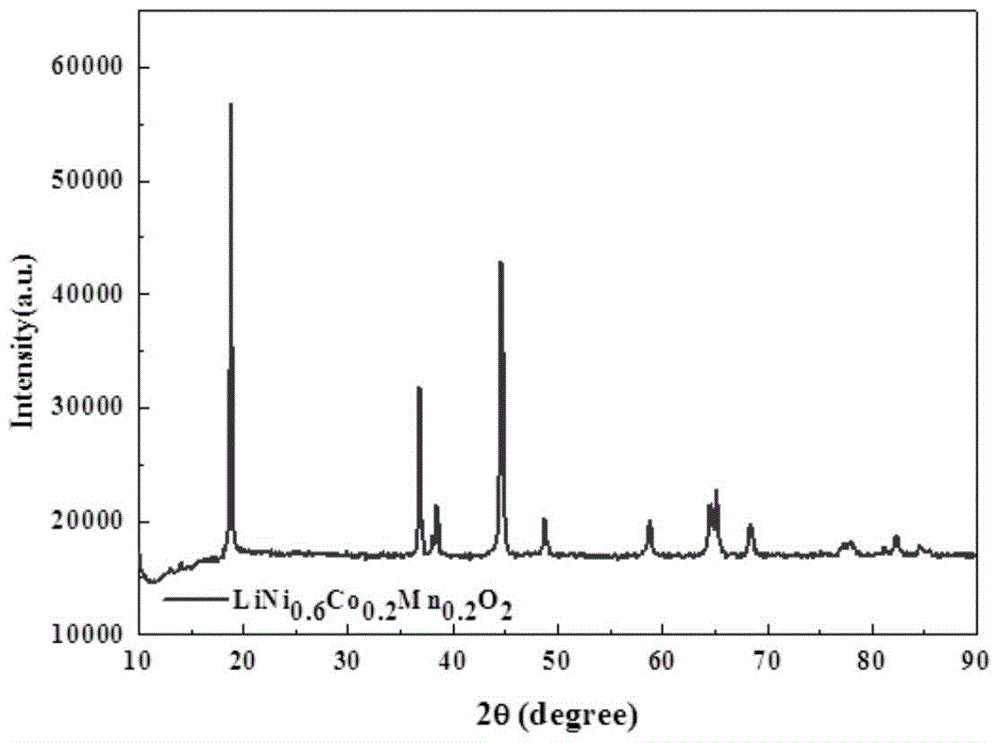
Preparation method of high-capacity, fast charge-discharge lithium-ion battery ternary cathode material
A technology for lithium-ion batteries and cathode materials, which is applied in battery electrodes, electrical components, secondary batteries, etc., can solve the problem that the cathode materials of ternary nickel-cobalt lithium manganese oxide lithium-ion batteries cannot be quickly charged. Putting down and other problems, to achieve the effect of low manufacturing cost, high tap density and good cycle performance
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0038] Weigh nickel sulfate, cobalt sulfate, and manganese sulfate respectively in a molar ratio of 6:2:2, and dissolve them in deionized water to prepare a total concentration of 2mol / L, pass an inert gas to remove oxygen for 30 minutes; prepare 4mol / L sodium carbonate as a precipitate Agent, 2mol / L ammonia water as complexing agent, through N 2 Remove oxygen for 40 minutes; prepare a 2mol / L ammonia solution as the bottom liquid and first add it to the reaction kettle, and pass N at the same time 2As a protective gas, under mechanical stirring, the nickel-cobalt-manganese salt solution is added to the reaction kettle at a rate of 2L / h by means of a metering pump, and the mixed solution of the precipitant and complexing agent is added dropwise, and the reaction system is precisely controlled The pH value is 8.0, the reaction temperature is 50°C, and the stirring speed is 500r / min. Co-precipitation reaction is carried out, and finally a brownish-yellow solid-liquid mixture is o...
Embodiment 2
[0047] Weigh nickel chloride, cobalt chloride, and manganese chloride respectively in a molar ratio of 6:2:2, and dissolve them in deionized water to prepare a total concentration of 1.5mol / L, and pass an inert gas to remove oxygen for 60 minutes; prepare 4mol / L L sodium hydroxide is used as precipitating agent, 2mol / L ammonia water is used as complexing agent, pass N 2 Remove oxygen for 60 minutes; prepare 2mol / L ammonia aqueous solution as the bottom liquid and first add it to the reaction kettle, and pass N 2 As a protective gas, under mechanical stirring, the nickel-cobalt-manganese salt solution is added to the reaction kettle at a dropping rate of 1L / h by means of a metering pump, and the mixed solution of the precipitating agent and complexing agent is added dropwise, and the reaction system is precisely controlled. The pH value is 11.5, the reaction temperature is 70°C, and the stirring speed is 1000r / min. Co-precipitation reaction is carried out, and finally a light b...
Embodiment 3
[0050] Weigh nickel nitrate, cobalt nitrate, and manganese nitrate at a molar ratio of 6:2:2, and dissolve them in deionized water to prepare a total concentration of 4mol / L, pass inert gas to remove oxygen for 45 minutes; prepare 8mol / L sodium bicarbonate As a precipitant, 10mol / L citric acid as a complexing agent, through N 2 Remove oxygen for 30 minutes; prepare 10mol / L citric acid aqueous solution as the bottom liquid and add it to the reaction kettle first, and pass N 2 As a protective gas, under mechanical stirring, the nickel-cobalt-manganese salt solution is added to the reaction kettle at a rate of 50L / h by means of a metering pump, and the mixed solution of the precipitant and complexing agent is added dropwise, and the reaction system is precisely controlled The pH value is 9, the reaction temperature is 60°C, and the stirring speed is 700r / min. Co-precipitation reaction is carried out, and finally a light brown solid-liquid mixture is obtained. After centrifugation...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Concentration | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com