Preparation method for 3,4,4,4-tetrafluoro-3-(trifluoromethyl)butyl-1-ol
A technology of trifluoromethyl and tetrahydrofuran, which is applied in the field of preparing 3,4,4,4-tetrafluoro-3-butan-1-ol, can solve the problems of high yield, poor surface activity, and insufficient production capacity, and achieve The principle and synthesis process are simple, the requirements for production equipment are low, and the effect of reaction conditions is low
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
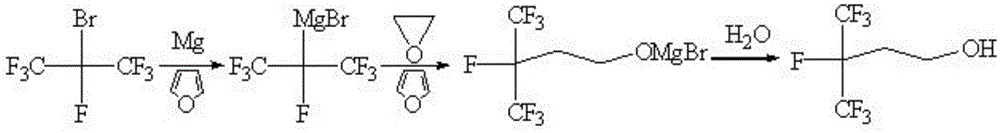
[0014] attached figure 1 The method for preparing 3,4,4,4-tetrafluoro-3-(trifluoromethyl)butan-1-ol according to the present invention:
[0015] First prepare the Grignard reagent, get 20ml of anhydrous tetrahydrofuran solvent and pour it into a three-neck flask, use nitrogen as a protective gas, add 0.022mol (0.528g) of magnesium powder, add a small amount of iodine as an initiator, and carry out magnetic stirring. 0.02 mol (4.98 g) of 2-bromoheptafluoropropane was added dropwise under heating conditions, and magnetically stirred for a period of time to obtain the Grignard reagent for use.
example 1
[0016] Example 1 (reaction temperature T=10 DEG C):
[0017] Take a four-neck bottle, take 50ml of tetrahydrofuran solvent, add 0.02mol (0.88g) of ethylene oxide, install a thermometer and a condensation reflux device, and control the temperature at 10°C, slowly add 0.03mol of Grignard reagent dropwise And carry out magnetic stirring reaction. After the dropwise addition, reflux reaction at 10°C for 0.5h. After the reaction, first heat up to evaporate the solvent, and then distill under reduced pressure at 80°C to distill the product 3,4,4,4-tetrafluoro- The mass of 3-(trifluoromethyl)butan-1-ol was distilled off to obtain 3.62 g, and the yield (calculated based on theoretical yield) was 84.50%.
[0018] Example 2-16, the reaction temperature is 10°C, 3,4,4,4-tetrafluoro-3-(trifluoromethyl)butan-1-ol, the raw materials and reagents used are as follows:
[0019] example
[0020] Reaction temperature / ℃
[0021] example
example 17
[0022] Example 17 (reaction temperature T=5°C):
[0023] Take a four-neck bottle, take 50ml of tetrahydrofuran as a solvent, add 0.02mol (0.88g) of ethylene oxide, install a thermometer and a condensation reflux device on the rack, control the system temperature at 5°C, and slowly add 0.03mol dropwise to prepare Grignard reagent and magnetic stirring reaction, after the dropwise addition, reflux reaction at 5°C for 0.5h. Tetrafluoro-3-(trifluoromethyl)butan-1-ol was distilled off to obtain a mass of 3.74 g, and the yield (calculated based on theoretical yield) was 87.37%.
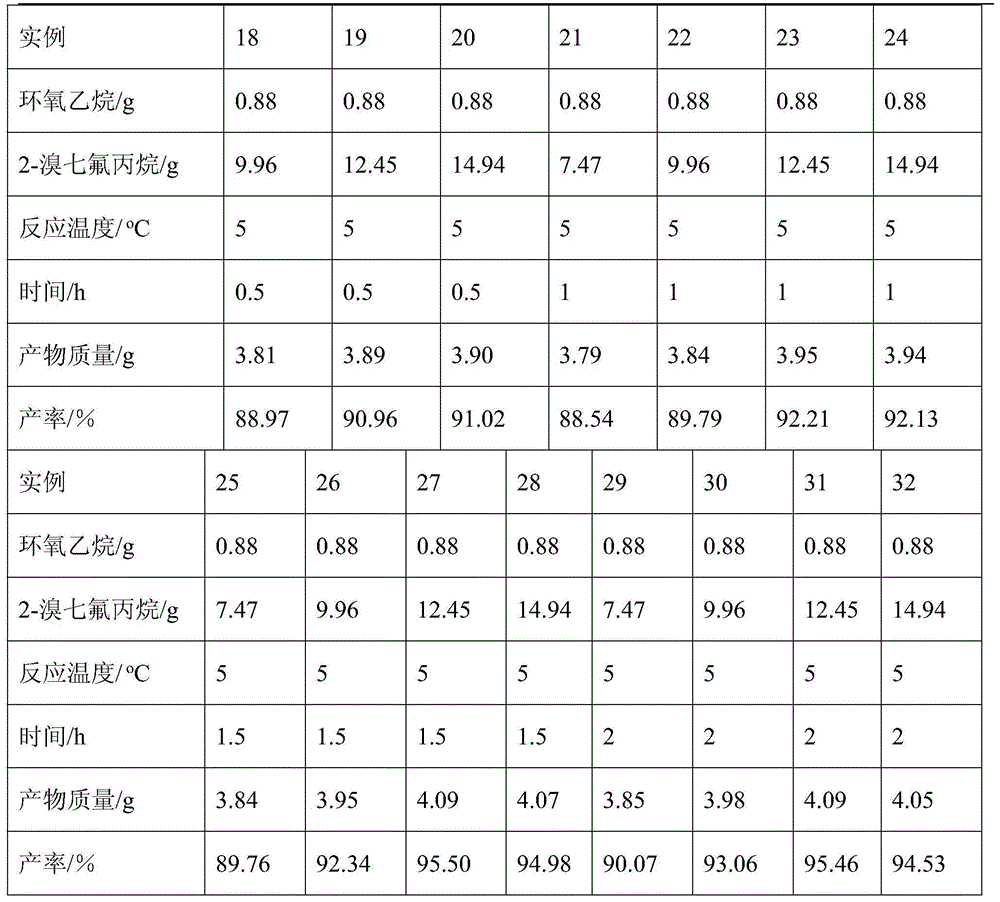
[0024] Examples 18-32, the reaction temperature is 5°C, 3,4,4,4-tetrafluoro-3-(trifluoromethyl)butan-1-ol, the raw materials and reagents used are as follows:
[0025]
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com