Erythromycylamine preparation method
A technology for erythromycin and a compound, which is applied in the field of preparing erythromycin, can solve the problems of relatively high pH requirements, difficult preparation and use, increased cost, etc., and achieves the effects of high product content, easy procurement, and convenient post-processing.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0045]
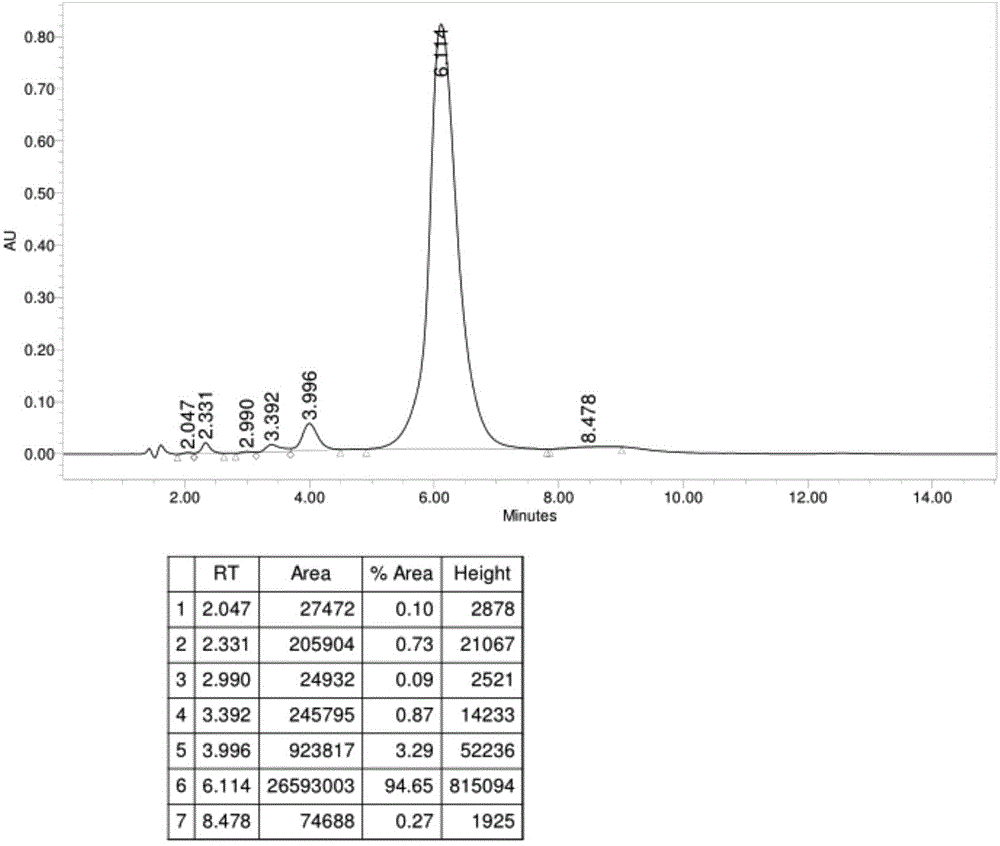
[0046] Dissolve 1 (3.99kg, 5.22mol) in methanol (20L), add hydrazine hydrate 80% aqueous solution (1.63kg 26.1mol), calcium oxide (1.31kg 23.37mol), stir at room temperature for 16h, then add Water, precipitated solid 2, suction filtered, and dried solid 2 at 40-50°C to obtain 4kg, yield 95%, purity 94.65%, see figure 1 .
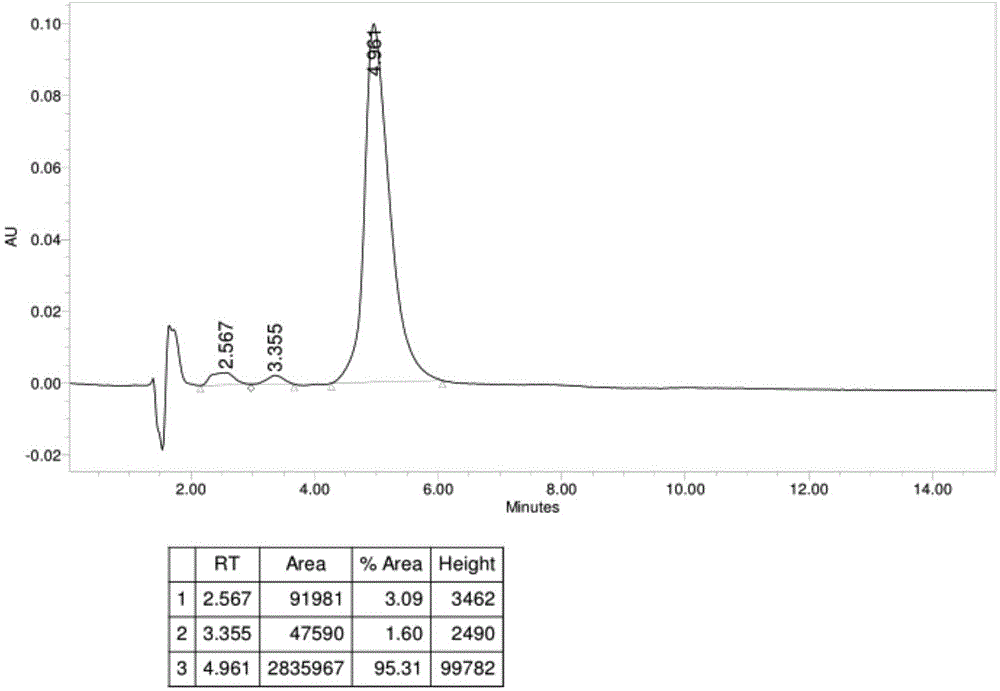
[0047] Dissolve 2 (4kg 4.96mol) in methanol (40L), add sodium nitrite (3.42kg 49.6mol) and water (4kg) in turn, and stir at room temperature for 30 minutes. Control -10°C, add 3N hydrochloric acid (20L) dropwise, and control the pH=2.5~3, adjust the pH=2.5~3 with a small amount of 1N hydrochloric acid after dropping, extract with dichloromethane (12L*2), use 15% hydrogen in the water phase Sodium oxide (8L) was used to adjust the pH to 9.2-9.8, extracted with L dichloromethane (12L*2), and the organic phase was washed once with water (2L) at an external temperature of 35°C, and concentrated until no distillate was produced to obtain 3. Cool do...
Embodiment 2
[0049]
[0050] Dissolve 1 (1kg, 1.31mol) in methanol (5L), add hydrazine hydrate 80% aqueous solution (0.39kg 7.86mol), calcium oxide (0.59kg 10.48mol), stir at room temperature for 16h, add water under rapid stirring, The solid 2 was precipitated, filtered with suction, and the solid 2 was dried at 40-50° C. to obtain 1 kg with a yield of 95% and a purity of 95%.
[0051]Dissolve 2 (1kg 1.24mol) in methanol (10L), add sodium nitrite (0.856kg 12.4mol) and water (1kg) in turn, and stir at room temperature for 30 minutes. Control -10°C, add 3N hydrochloric acid (5L) dropwise, and control the pH=2.5~3, adjust the pH=2.5~3 with a small amount of 1N hydrochloric acid after dropping, extract with dichloromethane (3L*2), use 15% hydrogen in the water phase Sodium oxide (2L) was used to adjust the pH to 9.2-9.8, extracted with dichloromethane (3L*2), the organic phase was washed once with water (2L) at an external temperature of 35°C, and concentrated until no distillate appeared ...
Embodiment 3
[0054]
[0055] Dissolve 1 (10kg, 13.1mol) in methanol (20L), add hydrazine hydrate 80% aqueous solution (4.1kg 65.5mol), calcium oxide (3.3kg 58.6mol), stir at room temperature for 16h, add water under rapid stirring, Solid 2 was precipitated, filtered with suction, and dried at 40-50° C. to obtain 10.02 kg with a yield of 95% and a purity of 95%.
[0056] Dissolve 2 (10.02kg 12.42mol) in methanol (100L), add sodium nitrite (12.8kg 186mol) and water (10kg) in turn, and stir at room temperature for 30 minutes. Control -10°C, add 3N hydrochloric acid (50L) dropwise, and control the pH=2.5~3, adjust the pH=2.5~3 with a small amount of 1N hydrochloric acid after dropping, extract with dichloromethane (30L*2), use 15% hydrogen in the water phase Sodium oxide (20L) was used to adjust the pH to 9.2-9.8, extracted with L dichloromethane (30L*2), the organic phase was washed once with water (5L) at an external temperature of 35°C, and concentrated until no distillate was produced t...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com