A method for hydrogen production by catalyzing the hydrolysis of ammonia borane with proton-responsive iridium complex
A technology for catalyzing ammonia borane and iridium complexes, applied in the field of energy and homogeneous catalysis, can solve the problems of poor water solubility of catalysts, and achieve good catalytic effect, good water solubility, and large hydrogen release effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
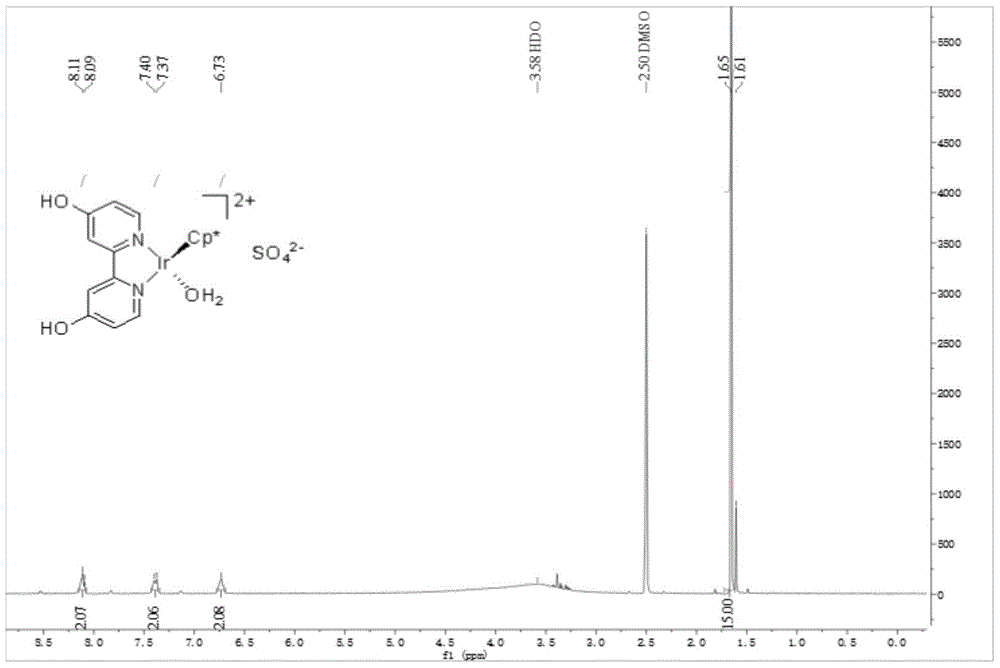
[0036] [Cp*Ir(6,6'-(OH) 2 -bpy)(OH 2 )] SO 4 Preparation and catalysis of ammonia borane hydrolysis for hydrogen production:
[0037] (1) NiBr 2 (PPh 3 )2 (1.1146g, 1.5mmol), newly activated Zn powder (0.4904g, 7.5mmol), Et 4 NI (1.2858g, 5mmol) and 2-chloro-6-methoxypyridine (0.7179g, 5mmol) were added to anhydrous and oxygen-free tetrahydrofuran (20mL), and stirred at 50°C for 47h under an inert gas atmosphere.
[0038] (2) After the reaction, the solvent was evaporated to dryness, ammonia water (2mol / L, 30mL) was added, dichloromethane (10mL) was dissolved, and the insoluble matter was filtered off. The filtrate was extracted three times, washed with saturated sodium chloride solution, dried over anhydrous sodium sulfate, and filtered. The solvent was removed by rotary evaporation, and the crude product was obtained as a light yellow solid. Isopropanol was added to the crude product for recrystallization, and white crystals were precipitated. After filtration, 6,6'-d...
Embodiment 2
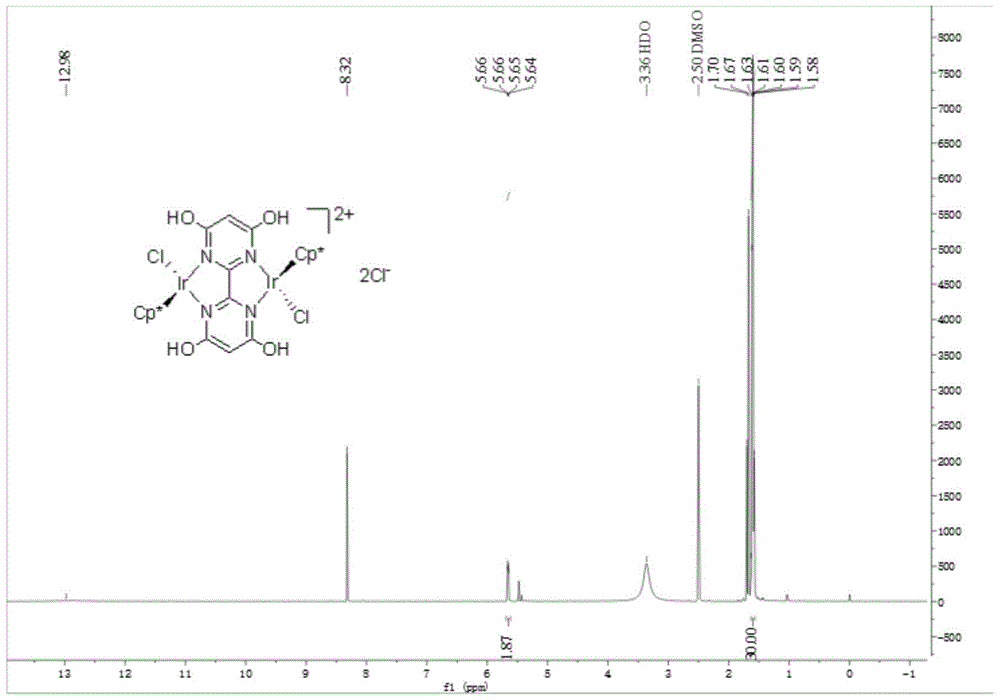
[0046] [{Cp*Ir(Cl)} 2 (thbm)]Cl 2 Preparation and catalysis of ammonia borane hydrolysis for hydrogen production:
[0047] (1) The synthesis of 4,4',6,6'-tetramethoxy-2,2'-bipyrimidine is the same as step (1) and (2) in Example 1
[0048] (2) Add 4,4',6,6'-tetrahydroxy-2,2'-bipyrimidine (0.1947g, 0.7mmol), iodotrimethylsilane (1mL, 7mmol) into anhydrous acetonitrile (5mL) , under an inert gas atmosphere, stirred and refluxed for 29h.
[0049] (3) After the reaction is finished, cool to room temperature. It was quenched by adding methanol, and the insoluble matter was collected by filtration, washed with methanol and ether successively to obtain a white solid. Dissolve and purify by heating with isopropanol, filter out insoluble matter, and dry to obtain 0.1181 g of pure product 4,4',6,6'-tetrahydroxy-2,2'-bipyrimidine, with a yield of 76%.
[0050] (4) Dissolve the ligand 4,4',6,6'-tetrahydroxy-2,2'-bipyrimidine (0.0444g, 0.2mmol) in anhydrous methanol, and then add [Cp*I...
Embodiment 3
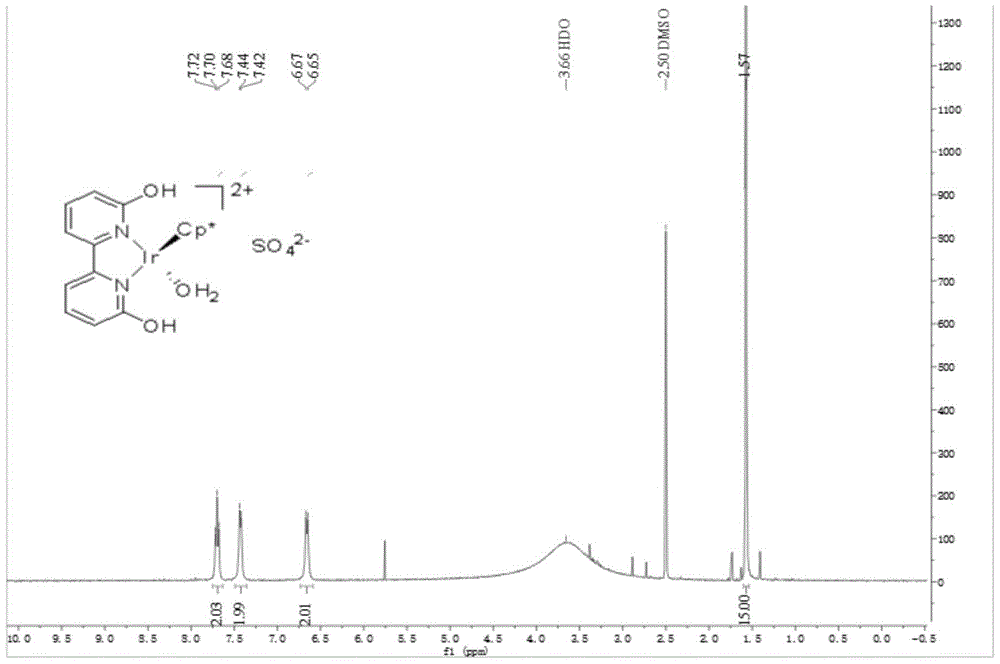
[0054] [Cp*Ir(th4bpym)(OH 2 )] SO 4 Preparation and catalysis of ammonia borane hydrolysis for hydrogen production:
[0055] (1) The preparation of 2,2',6,6'-tetrahydroxy-4,4'-bipyrimidine is the same as steps (1), (2) and (3) in Example 2.
[0056] (2)[Cp*Ir(th4bpym)(OH 2 )] SO 4 The preparation is the same as step (6) in Example 1.
[0057] (3) Carry out deoxidation treatment to catalyst, deionized water, with embodiment 1 step (7).
[0058] (4) Under the protection of inert gas, take ammonia borane (30.8 mg, 1 mmol), add 10 mL of deoxygenated deionized water with pH=9 into the bottle, and heat to 65° C. under the protection of nitrogen. Catalyst solution (0.2 mL, 1 μmol) was added, the reaction was stirred, and the gas was collected by drainage. Record the hydrogen release volume and reaction time, calculate the TON of the catalytic reaction based on the hydrogen release amount is 1000, and the TOF of the initial 5min is 1230h -1 .
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com