Method for marking protein by carboxyl microsphere
A technology for labeling proteins and microspheres, which is applied in the direction of material inspection products, measuring devices, instruments, etc., can solve problems such as harsh reaction conditions, wasting antibodies, and affecting coupling efficiency, achieving high-efficiency coupling, reducing dosage, and avoiding hydrolysis Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0050] Example 1 Carboxyl latex microspheres labeled cystatin C antibody
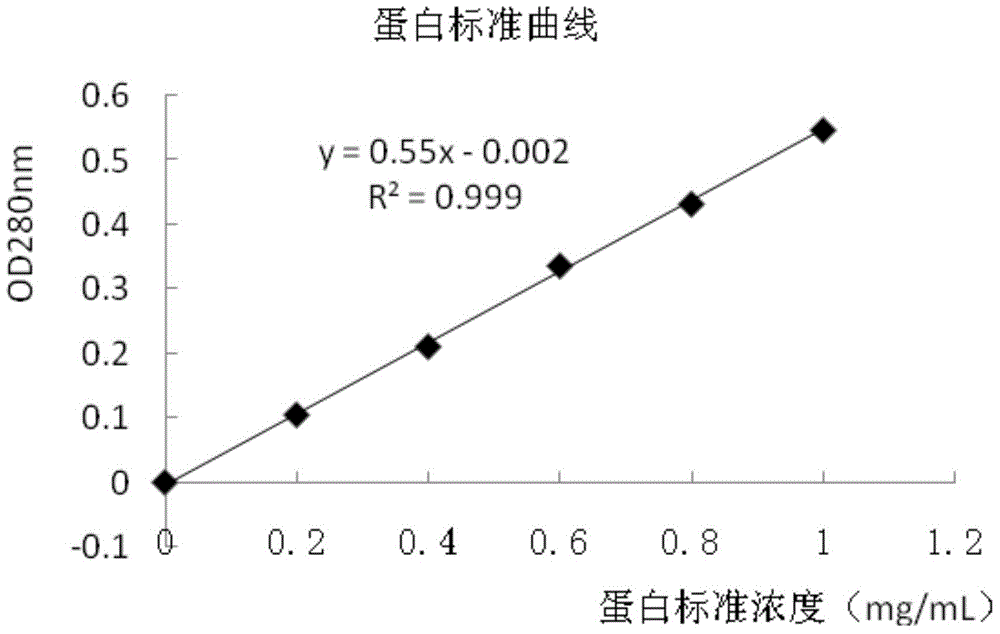
[0051] Cystatin C antibody is a product of Oriental Yeast Company, polyclonal antibody, the concentration is 15mg / mL. The antibody only reacts with human cystatin C and has no cross-immune reaction with other antigens, which basically meets the requirements of this experiment. The latex microspheres are products of JSR Company, the particle size is 61 nm, the carboxyl content is 0.190 mmol / g, the concentration is 5.2%, and the solution is purified water with 0.09% sodium azide. EDC and NHS are products of Pierce Company.
[0052] Latex microspheres are prepared into 0.5% solution with 10mM MES, the activation solution of pH 6.00, stir evenly, according to the carboxyl group content on the latex particle surface, according to the ratio of molar ratio EDC:NHS:-COOH=1:1:1, calculate all The required amount of EDC and NHS was weighed and directly added to the latex microsphere solution, stirred while addi...
Embodiment 2
[0058] The preparation and application of the assay kit of embodiment 2 cystatin C
[0059] (1) Prepare R2 reagent.
[0060] The latex particles (i.e. the carboxyl microspheres labeled cystatin C antibody prepared in Example 1) connected to the cystatin C antibody were washed with 10mM Tris, 0.1%BSA, 0.1%NaN 3 , Dilute the pH7.50 solution to 0.05% to make R2 reagent.
[0061] (2) Prepare R1 reagent.
[0062] Add 2.5% NaCl and 0.5% Tween-20 to a buffer solution with a final concentration of 2.5% and 0.5% Tween-20 in 10 mM Tris buffer solution with a pH value of 8.5, and stir evenly to obtain reagent R1.
[0063] (3) Preparation of standard products
[0064] Dissolve pure cystatin C in 10 mM Tris, 0.1% BSA, 0.1% NaN 3 , in a pH 7.50 solution, make standard products with gradient concentrations of about 0, 0.5, 1, 2, 4, and 8 mg / L.
[0065] Reagents R1, R2 and standards constitute a cystatin C assay kit.
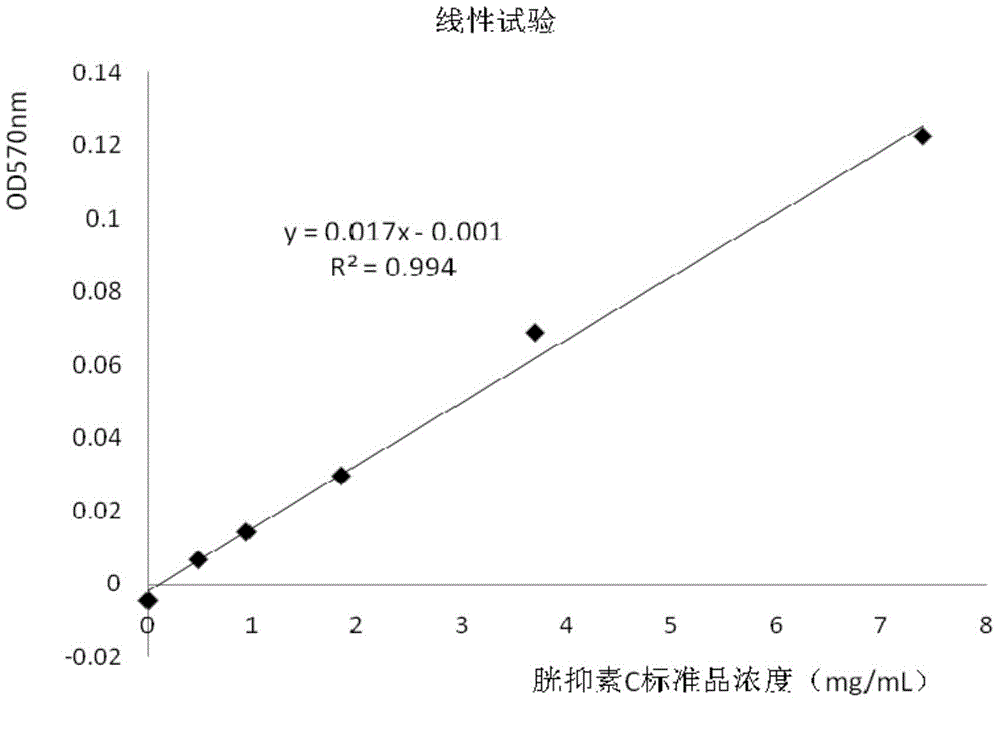
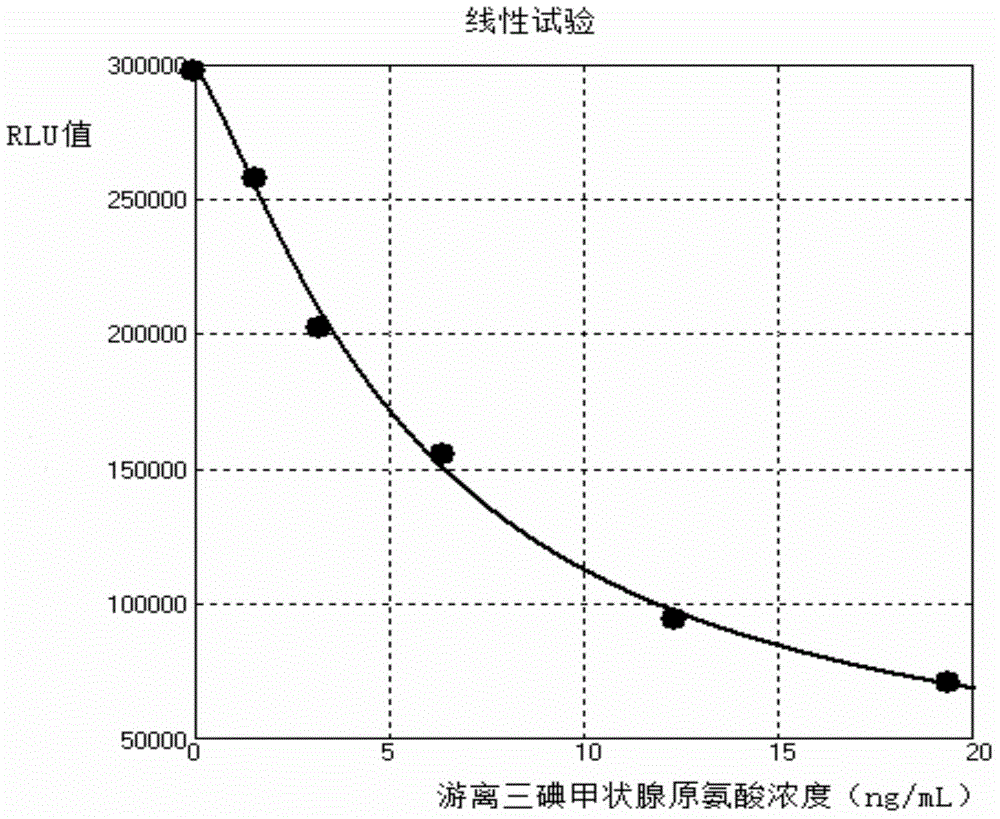
[0066] (4) Linearity test of cystatin C assay kit detection
[0067...
Embodiment 3
[0073] Example 3 Carboxyl Magnetic Microspheres Coupling Streptavidin
[0074] The magnetic microspheres are products of Merck Company, the particle size is 0.5-2 μm, the carboxyl content is 78 μmol / g, the concentration is 10%, and the solution is purified water with 0.09% sodium azide; Dry powder; EDC and NHS are products of Pierce Company.
[0075] The magnetic microspheres are prepared into a 1% solution with 20mM MES, pH 5.80 activation solution, stirred evenly, according to the carboxyl content on the surface of the magnetic microspheres, according to the ratio of molar ratio EDC:NHS:-COOH=1:1:1, calculate The required amount of EDC and NHS was weighed and directly added to the magnetic microsphere solution, stirred while adding, and stirred at room temperature for 15 minutes. Immediately use NaOH to adjust the pH value of the magnetic microsphere solution to 8.5, then add streptavidin dissolved in purified water in advance to make the final concentration of streptavidin...
PUM
| Property | Measurement | Unit |
|---|---|---|
| diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com