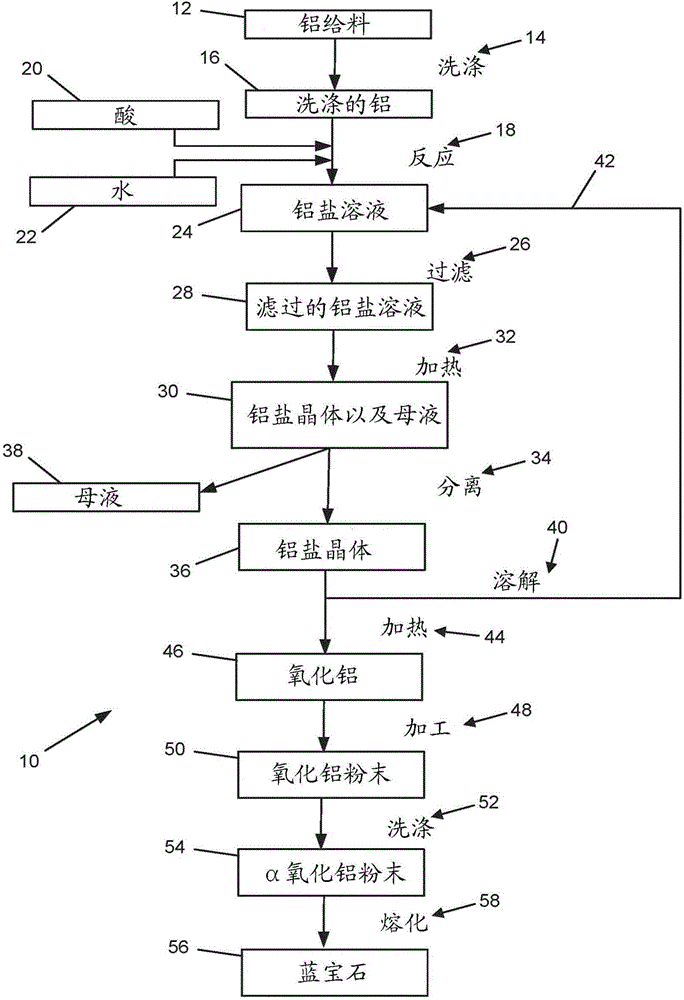
Process for making high-purity aluminum oxide
A high-purity alumina technology, applied in chemical instruments and methods, alumina/aluminum hydroxide, aluminum halide, etc., can solve problems such as out-of-control reactions and increased costs
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0060] Provides 99.99+% Aluminum 656g. Aluminum comprises the following components: 14.1 ppmw Si, 17.1 ppmw Fe, 0.04 ppmw Mn, 0.14 ppmw Mg, 27.8 ppmw Cu, 0.14 ppmw Ti, 0.29 ppmw Zn, 0.02 ppmw Cr, 0.24 ppmw V, 0.23 ppmw Ga, and the remainder being aluminum. Aluminum was added to 300 g of distilled water and 150 g of reagent grade HCl. Distilled water contains less than 0.5mg / L Si, less than 1mg / L Na, less than 1mg / L K, less than 1mg / l Ca, less than 1mg / L Mg, less than 0.5mg / L Al, less than 0.1mg / L L Fe, less than 0.1mg / L P, and less than 0.5mg / L Cl. Reagent grade HCl contains less than 1ppmw SO 4 , less than 1ppmw SO 3 , less than 1ppmw free Cl, less than 3ppmw NH 4 , less than 0.01ppmw As, less than 1ppmw heavy metals, less than 0.2ppmw Fe, and less than 5ppmw extractable organic matter.
[0061] A liquid mixture of aluminum, water and HCl was heated above 65°C in a high density polyethylene (HDPE) container for 24 hours. After heating, an additional 150 g of reagent gr...
Embodiment 2
[0066] Provides 99.99+% aluminum 247g with the following composition: 7.5ppmw Si, 3.9ppmw Fe, 0.05ppmw Mn, 2.7ppmw Mg, 2.7ppmw Cu, 0.07ppmw Ti, 1.5ppmw Zn, 0.02ppmw Cr, 0.13 ppmw V, 0.15ppmw Ga, and the remainder Al. Aluminum was added to 900 g of reverse osmosis treated and distilled water. Water and aluminum were heated to above 85°C in a polypropylene container and 999 g of reagent grade HCl with a concentration of 36% was added to the container. The aluminum, water, and HCl were allowed to remain for more than 48 hours. The vessel was vented to the condenser and then to atmosphere. It was determined that 102g of aluminum was dissolved in the liquid.
[0067] The liquid was then drained from the container and filtered through a 1 μm polypropylene filter. The resulting liquid is 1865g with a specific gravity of 1.28g / cm 3 polyaluminum chloride (PAC). The PAC liquid was then heated to 125°C in a high temperature resistant polyvinylidene fluoride (PVDF) vessel for 24 hou...
Embodiment 3
[0070] Provides 99.99+% aluminum 30.5 kg with the following composition: 7.5 ppmw Si, 3.9 ppmw Fe, 0.05 ppmw Mn, 2.7 ppmw Mg, 2.7 ppmw Cu, 0.07 ppmw Ti, 1.5 ppmw Zn, 0.02 ppmw Cr, 0.13ppmw V, 0.15ppmw Ga, and the remainder being Al. Aluminum was added to 80 L of reverse osmosis treated and distilled water. The aluminum surface was cleaned with HCl before adding to water. Water and aluminum were heated to above 80°C in a polypropylene container. After heating, 120 L of HCl with a concentration of 38% was added to the vessel. HCl contains less than 1 ppmw of each of Fe, Na, Si, Ca, Mg, and Zn. The mixture of aluminum, water and HCl was allowed to stand for over 92 hours. The vessel was vented to the condenser and then to atmosphere. The concentrated liquid is recycled and reused in future batches.
[0071] It was determined that 16Kg of aluminum was dissolved in water and HCl mixture to form a polyaluminum chloride (PAC) solution. The solution was drained from the reactio...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Melting point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com