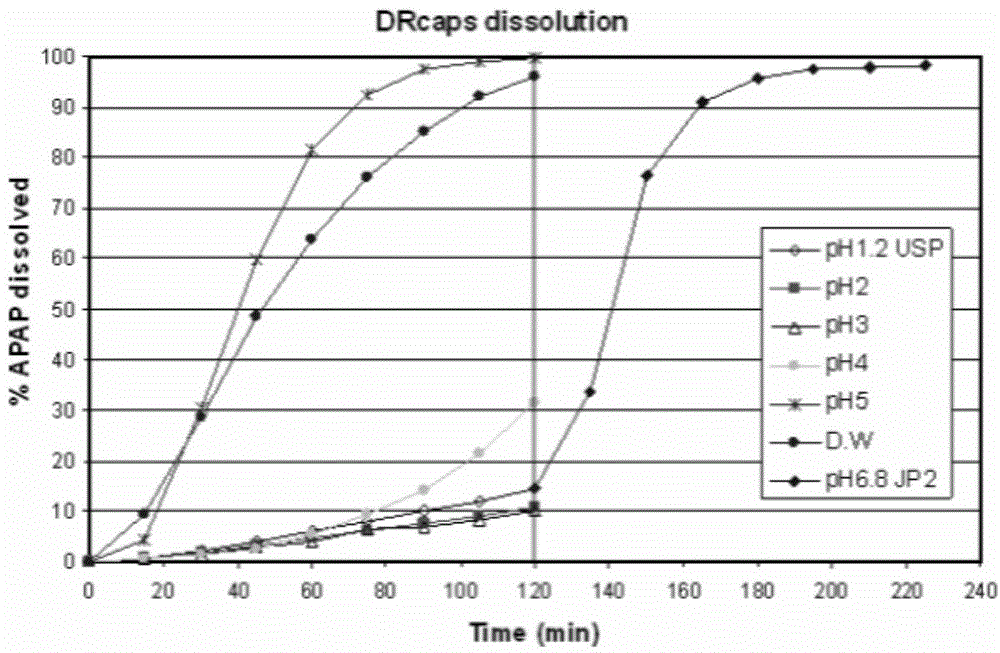
Fecal microbiota capsule, as well as preparation method and application thereof
A fecal bacteria and capsule technology, which is applied to the fecal bacteria capsules and the fields of preparation and application thereof, can solve the problems of short storage time, limitation, inconvenient access, etc., and achieve the effects of increasing safety and improving freeze-drying survival rate.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0064] To prepare fecal bacteria capsules:
[0065] Method 1: Put about 120 g of fresh feces samples from the donor in a blender, add 250 ml of sterile 0.9% NaCl solution for preliminary homogenization; filter the fecal slurry through a stainless steel filter to remove large particles; put it on ice Transported to the laboratory and processed within 2 hours after collection; samples were weighed in a nitrogen bioengineering kitchen and further homogenized in a homogenizer. The slurry was filtered step by step through molecular sieves with diameters of 2.0mm, 1.0mm, 0.4mm and 0.1mm to remove unabsorbed food residues and small particulate matter; the re-filtered samples were centrifuged at 3000 rpm for 10min, and the sediment Resuspend in 100ml of 0.9% NaCl solution, disperse and resuspend. Drop from 25°C to -80°C through the "two-step method": the first step is to cool down at a rate of 1-2°C / min; the second step is to cool down at a rate of 3-5°C / min; the total pre-freezing t...
Embodiment 2
[0079] The fecal bacteria capsule prepared by embodiment 1 method 3~5 is carried out clinical trial:
[0080] Requirements for donors:
[0081] 1) Obtain the informed consent of the fecal donor and recipient before FMT.
[0082]2) Donors are mainly selected from individuals who have a close relationship with the recipient, such as husband and wife, parents, children and close friends. This is related to the willingness of the recipients. At the same time, this part of the population has a similar micro-ecological environment to the recipients, which may theoretically have a more positive effect on FMT. Gough et al. believed that the use of feces from relatives or friends of recipients was more effective than feces from unrelated donors, and pointed out that gender differences between donors and recipients had little effect on disease remission.
[0083] 3) Donor inclusion criteria:
[0084] ①No known infectious diseases;
[0085] ② No antibacterial drugs or other drugs tha...
Embodiment 1
[0117] Embodiment 1 method 3~5.
[0118] 4. Standardized program for transplantation:
[0119] ①Recipients stopped using antibacterial drugs 3 days before transplantation;
[0120] ②Recipients, regardless of the transplantation route, should prepare for intestinal cleansing 1 day before;
[0121] ③Give metoclopramide, an anti-motor function drug, to facilitate intestinal retention of fecal fluid;
[0122] ④ Proton pump inhibitors should be given the night before and in the morning of FMT to inhibit the secretion of gastric acid.
[0123] ⑤ Within 2 days after transplantation, closely observe the patient's abdominal pain and diarrhea, and observe the patient's body temperature. If there are transient adverse reactions, such as elevated body temperature and transient increase in the number of diarrhea, symptomatic treatment should be taken.
[0124] 5. Standardized capsule resuscitation and administration process
[0125] ①A total of 12 capsules, given in two days, 6 capsule...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com