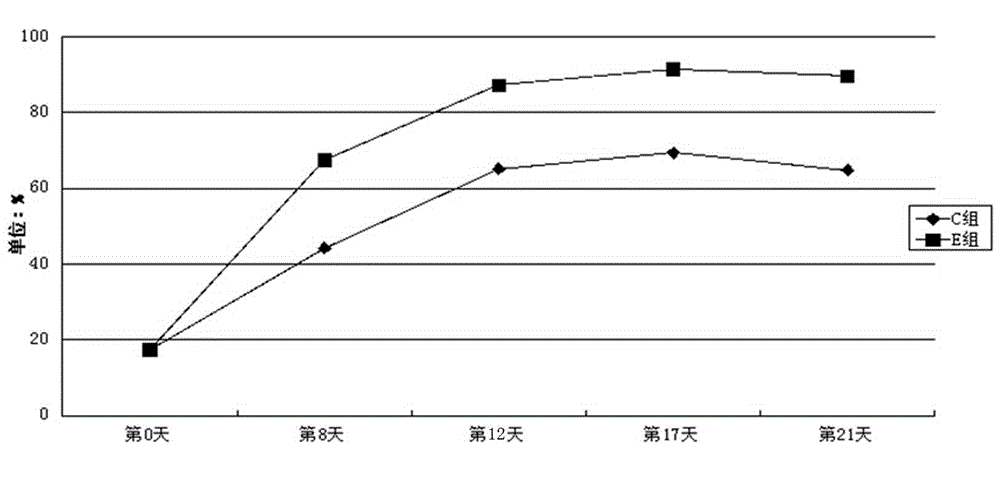
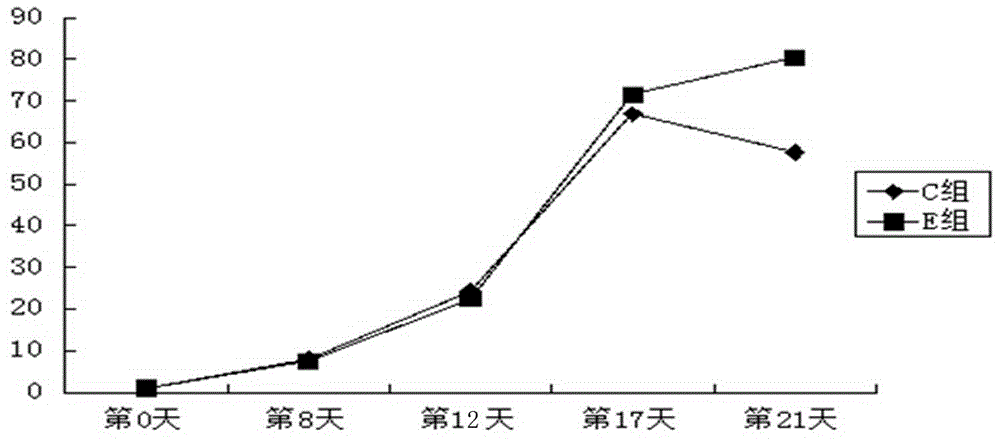
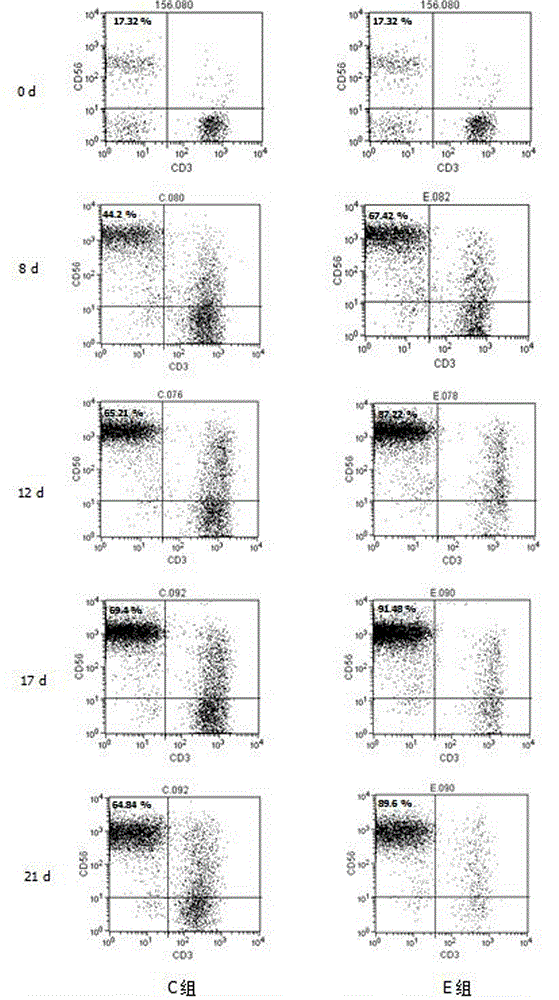
Solid tumor patient autologous NK cell separation, excitation, amplification and activity detection method
A technology for NK cells and solid tumors, applied in biochemical equipment and methods, animal cells, vertebrate cells, etc., to achieve the effects of multiple amplification, simple operation steps, and low cost of reagents and consumables
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0071] A method for the isolation, activation and expansion of autologous NK cells from patients with solid tumors, comprising the following steps:
[0072] (1) Collection and isolation of NK cells:
[0073] 1.1. Peripheral blood collection: 40-60ml of peripheral blood from patients with solid tumors, preferably 50ml. The collection device is a disposable 100ml syringe, which is filled with 10ml of normal saline and 50unit / ml of heparin sodium anticoagulant, and the dosage of heparin sodium is calculated according to the volume of blood collected. Take 2ml of peripheral blood and detect lymphocyte subsets by flow cytometry.
[0074] 1.2. NK cell separation:
[0075] 1.2a. Put the collected peripheral blood into a 50ml centrifuge tube, centrifugation conditions: centrifugal force 500g, centrifugation time 10min. Aspirate the plasma with a straw and put it into another centrifuge tube, inactivate it in a water bath at 56°C for 30 minutes and then centrifuge. Centrifugation co...
Embodiment 2
[0106] A method for detecting cytotoxicity of autologous NK cells of patients with solid tumors, comprising the steps of:
[0107] 3.1. CFSE labeling target cells:
[0108] Tumor cell culture: suspend the tumor cell line (K562 cells) in 1640 medium containing 10% inactivated fetal bovine serum (FBS), and place at 37°C, 5% CO 2 , subcultured under saturated humidity conditions.
[0109] CFSE-labeled target cells: collect tumor cells in good growth condition, use PBS buffer, centrifuge at 300g centrifugal force, and centrifuge at room temperature for 5min. Wash twice, count with trypan blue and resuspend cells with PBS buffer. Add the pre-warmed CFSE-PBS solution into the cell mass (the final concentration of CFSE is 2umol / L), gently blow and beat the suspended cells (cell concentration 1×10 6 / mL). Place the cells at 37°C for 5-10min. Add 10 mL of 1640 medium containing 10% FBS to terminate the reaction. Centrifuge the cells, add 10 mL of 1640 medium containing 10% FBS, pla...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com