Method for determining PEG content in biological sample
A determination method and technology for biological samples, applied in the field of determination of PEG content in biological samples, can solve the problems of reduced quantitative accuracy, no fundamental solution to the problem, and increased operation steps, so as to improve sensitivity and accuracy, and improve exclusive The effect of discernment
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
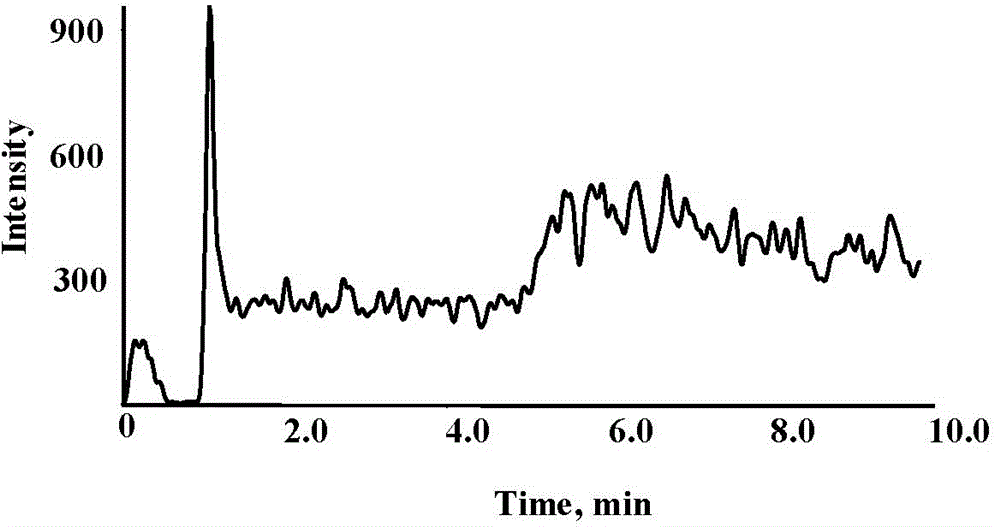
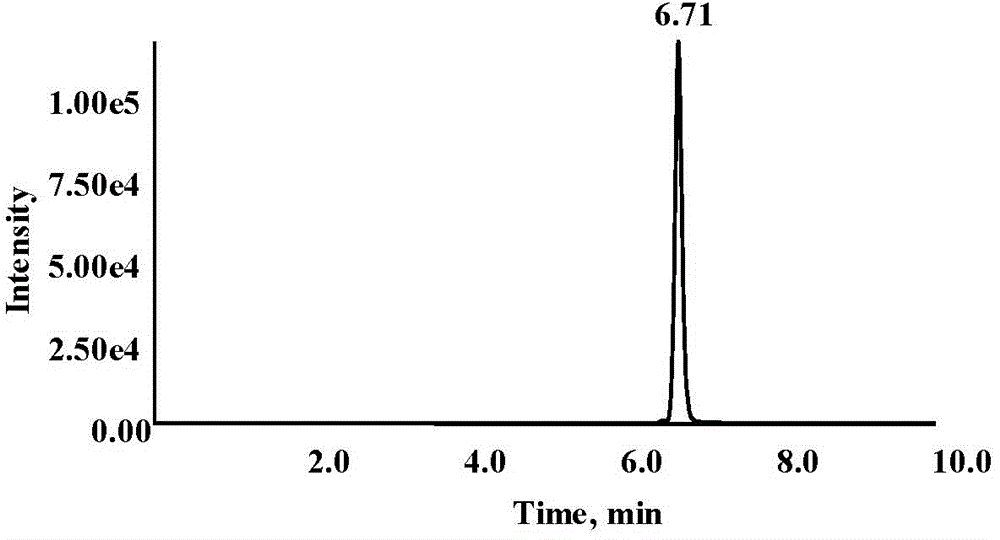
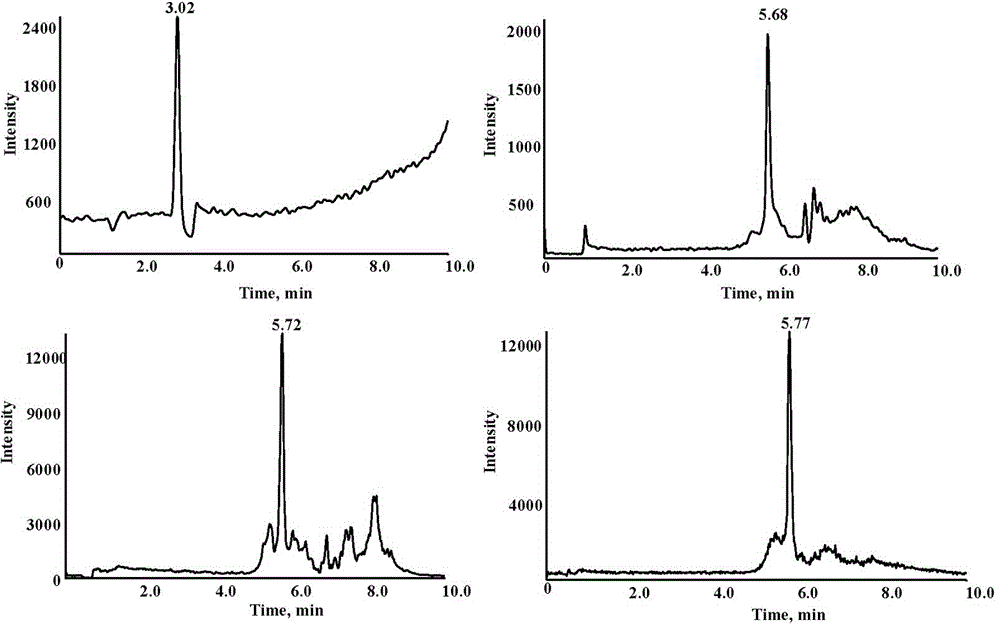
Image
Examples
Embodiment 1
[0038] A method for determining PEG content in a biological sample, which is determined by liquid chromatography-time-of-flight mass spectrometry (LC-Q-Q-TOF), and the determination steps include:
[0039] A. Establish a standard curve for PEG determination;
[0040] B. Use liquid chromatography-time-of-flight mass spectrometry to measure the sample to be tested, and calculate the concentration of PEG in the sample to be tested by the standard curve obtained in step A;
[0041] The chromatographic conditions and mass spectrometry conditions of steps A and B are the same, wherein the mass spectrometry conditions are based on triple tandem mass spectrometry, and the settings of the mass spectrometry part of steps A and B are: the voltage in the first mass analyzer Q1 does not select precursor ions, charged particles After all passes, enter the second mass analyzer Q2; set the collision energy in the second mass analyzer Q2 to break the charged particles into fragment ions; selec...
Embodiment 2
[0055] On the basis of Example 1, the determination of PEG 4000 in plasma was specifically selected for quantification.
[0056] The preparations before the measurement are:
[0057] Collection of Rat Plasma Samples
[0058] ① Select one male rat with a body weight of 200 g ± 10 g, free to eat and drink;
[0059] ② Weigh PEG 4000 and dilute to 1.0 mg / mL with normal saline;
[0060] ③The administration process is as follows: tail vein injection administration, the administration dose is 0.6 mL of 1.0 mg / mL PEG 4000 normal saline solution per mouse. Rats were treated before administration (0 h) and after administration at 0.083 h, 0.25 h, 0.5 h, 0.75 h, 1 h, 2 h, 3 h, 4 h, 6 h, 8 h, 10 h, 12 h , 24 h, take 0.5 mL of blood through the orbit of the rat, put the whole blood in a pre-cooled heparinized EP tube, centrifuge at 4°C (13300 rpm, 5 min), separate and transfer all the plasma to another EP tube Stored in a -20°C freezer for testing.
[0061] Determination of PEG 40...
Embodiment 3
[0091] The determination of the content of PEG 10000 in the plasma sample is the same as in Example 2 except that the following parameters are different from Example 2.
[0092] In the preparatory work before the determination, the rats were injected with PEG 10000 saline solution, and the standard product selected when making the standard solution was PEG 10000. Among the determination parameters: the unclustering voltage in the mass spectrometry condition is 100 V, the collision voltage is 30 eV; the chromatographic elution program is shown in Table 6. See Tables 7 and 8 for standard curves and accuracy.
[0093]
[0094]
[0095]
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com