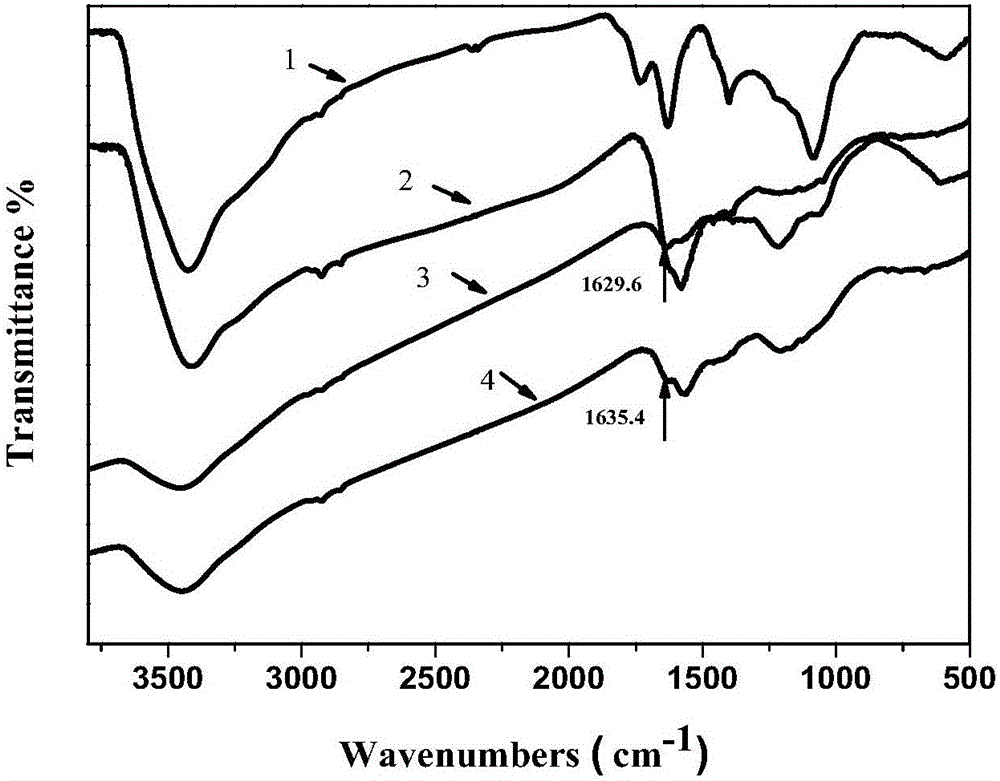
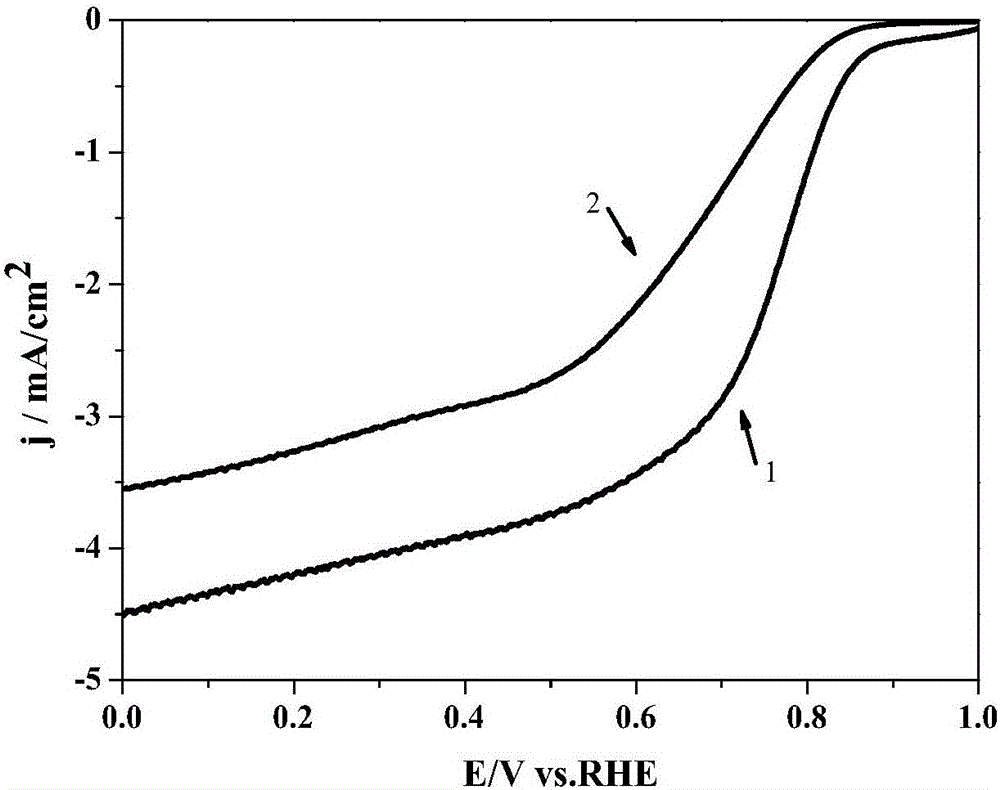
Preparing method for non-precious metal ion complexation Schiff base graphene catalyst
A non-noble metal and graphene technology, applied in the field of preparation of non-noble metal ion-complexed Schiff base graphene catalysts, can solve the problems of low catalytic activity, weak mass transfer inside the catalyst, poor conductivity between graphene layers, etc., to achieve The operation process is simple, the production cost is low, and the method is simple and easy to implement
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0032] (1) Preparation of graphene oxide
[0033]Take by weighing 3 grams of graphite powder, join 600 milliliters of concentrated phosphoric acid: concentrated sulfuric acid volume ratio is in the mixed solution of 1: 9, after mixing uniformly, obtain graphite powder mixed solution; After above-mentioned graphite powder mixed solution is placed in ice-water bath, Slowly add 18 grams of potassium permanganate solid powder, stir and mix evenly to obtain a potassium permanganate graphite powder mixture; then raise the temperature of the potassium permanganate graphite powder mixture to 50°C, stir for 12 hours, and then slowly pour Excessive ice water to obtain a purple-green ice water solution; finally, slowly and continuously add hydrogen peroxide solution with a mass fraction of 30% to the above-mentioned purple-green ice water solution until the solution turns golden yellow and stop adding, after filtration, dilute hydrochloric acid and ultrapure After alternate washing with ...
Embodiment 2
[0041] Step (1) is the same as step (1) in Example 1.
[0042] (2) Preparation of aminated graphene
[0043] Graphene oxide: p-phenylenediamine mass ratio is 1: 140 and weighs the graphene oxide and p-phenylenediamine prepared by step (1); first disperses the graphene oxide prepared by step (1) in deionized water , forming a graphene oxide suspension with a mass concentration of 0.8 mg / ml; then adding p-phenylenediamine to the above-mentioned graphene oxide dispersion, and stirring at room temperature for 15 minutes to form a p-phenylenediamine graphene oxide suspension; then The above p-phenylenediamine graphene oxide suspension was heated to 80°C, stirred and refluxed for 4 hours; finally, aminated graphene was obtained after suction filtration, ethanol washing 4 times, and drying;
[0044] (3) Preparation of Schiff base graphene
[0045] According to the mass ratio of aminated graphene: melamine: salicylaldehyde 1: 4.9: 19.1, respectively weigh the aminated graphene, mela...
Embodiment 3
[0051] Step (1) is the same as step (1) in Example 1.
[0052] (2) Preparation of aminated graphene
[0053] Be that 1: 186 by graphene oxide: melamine mass ratio take the graphene oxide prepared by step (1) and melamine; First the graphene oxide prepared by step (1) is dispersed in deionized water to form a mass concentration of 1.2 mg / ml of graphene oxide suspension; then melamine was added to the above-mentioned graphene oxide dispersion, and stirred at room temperature for 20 minutes to form a melamine graphene oxide suspension; then the above-mentioned melamine graphene oxide suspension was heated to 100°C, Stir and reflux for 5 hours; finally obtain aminated graphene after suction filtration, washing with ethanol for 5 times, and drying;
[0054] (3) Preparation of Schiff base graphene
[0055] According to the mass ratio of aminated graphene: melamine: salicylaldehyde 1: 10: 39, take respectively the aminated graphene, melamine and salicylaldehyde prepared by step (2)...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com