Rabeprazole sodium enteric-coated pellet capsule and preparation method thereof
A rabeprazole sodium enteric and rabeprazole sodium technology, which is applied in the field of enteric-coated pellets and capsules and its preparation, can solve the problems of rabeprazole sodium degradation, no stabilizer added to the drug layer, unreasonable medium, etc. problems, to achieve the effect of improving acid resistance, superior stability, and superior acid resistance
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
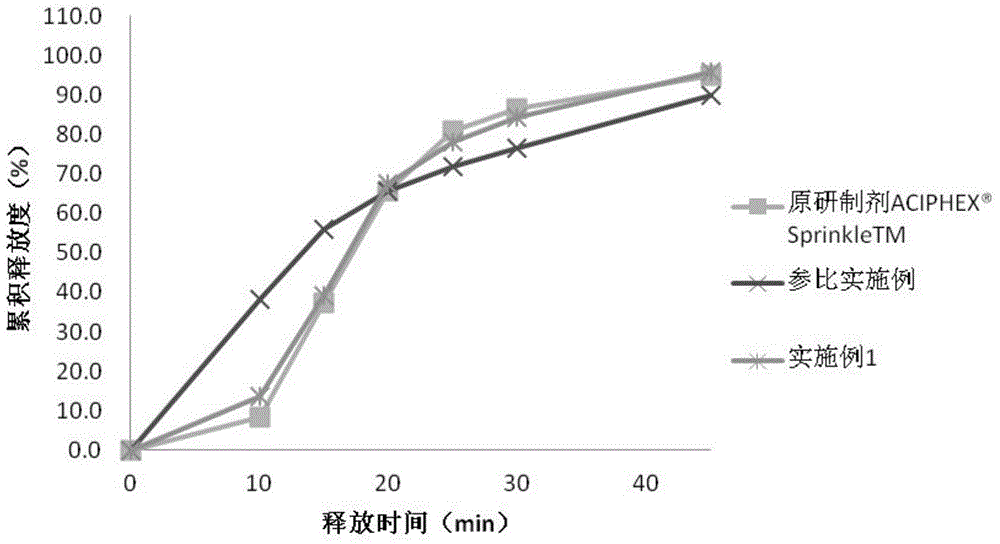
Embodiment 1
[0042] Embodiment 1 Rabeprazole sodium enteric-coated pellet capsule and preparation method thereof
[0043] The formula composition of the rabeprazole sodium enteric-coated pellets and capsules of this embodiment is shown in Table 1 below.
[0044] Table 1. Formula of rabeprazole sodium enteric-coated pellets and capsules
[0045]
[0046] The preparation method of the rabeprazole sodium enteric-coated pellets of the present embodiment comprises the steps:
[0047] A. Preparation of drug-layered pellets
[0048] Weigh 36 g of hypromellose, add 100 g of purified water and stir to dissolve. Take another 500g of purified water, add 5g of magnesium oxide, emulsify for 5min, and add to the hypromellose solution, add 5g of sodium hydroxide, stir to dissolve. While stirring, add 100.0 g of rabeprazole sodium, stir to dissolve, and obtain the coating solution of the drug layer. Put 500g of blank sugar pills in a fluidized bed for coating, the fan frequency is 16-20Hz, the air ...
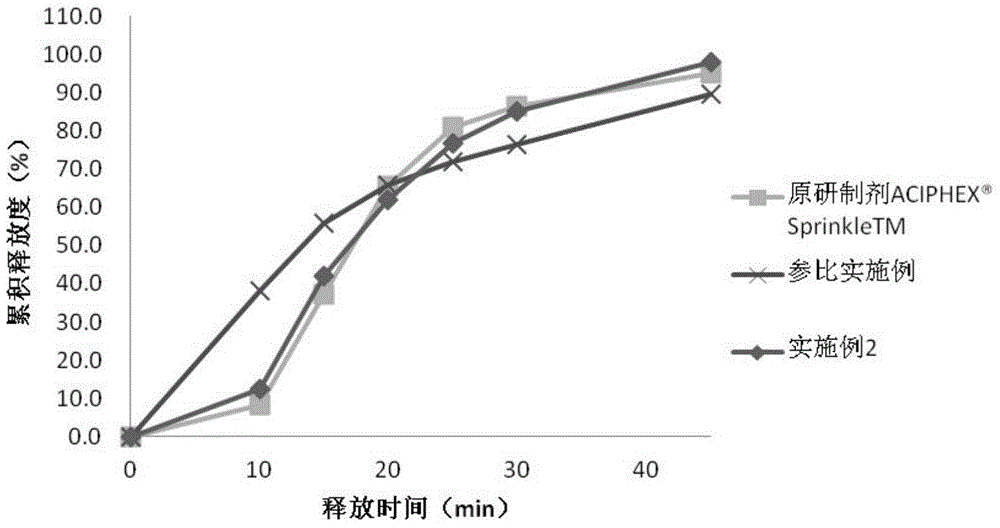
Embodiment 2
[0055] Embodiment 2 Rabeprazole sodium enteric-coated pellets capsule and preparation method thereof
[0056] The prescription composition of the rabeprazole sodium enteric-coated pellets and capsules of this embodiment is shown in Table 2 below.
[0057] Table 2. Formula of rabeprazole sodium enteric-coated pellets and capsules
[0058]
[0059]
[0060] The preparation method of the rabeprazole sodium enteric-coated pellet capsule of this embodiment is the same as that of embodiment 1.
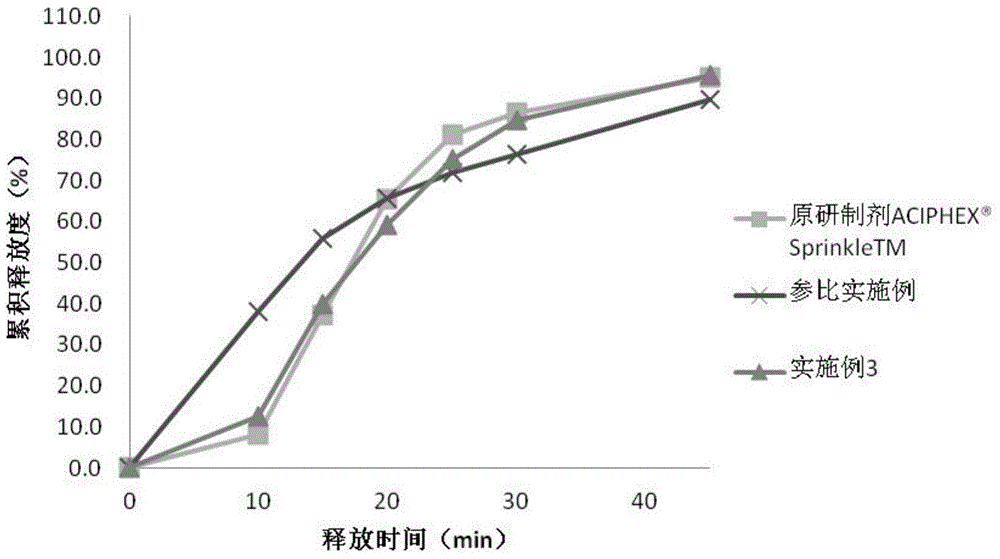
Embodiment 3
[0061] Embodiment 3 rabeprazole sodium enteric-coated pellet capsule and preparation method thereof
[0062] The prescription composition of the rabeprazole sodium enteric-coated pellets and capsules of this embodiment is shown in Table 3 below.
[0063] Table 3. Formula of rabeprazole sodium enteric-coated pellets and capsules
[0064]
[0065] The preparation method of the rabeprazole sodium enteric-coated pellet capsule of this embodiment is the same as that of embodiment 1.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com