Synthesizing method of n-alkyl-beta-D-glucopyranoside
A technology of glucopyranoside and fully acetylated glucopyranose, applied in the field of synthesis of n-alkyl-β-glucoside, which can solve the problems of physical injury to experimenters, unavoidable environmental pollution, cumbersome preparation process, etc. Effects of low cost, reduced bodily harm, and simplified synthesis process
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
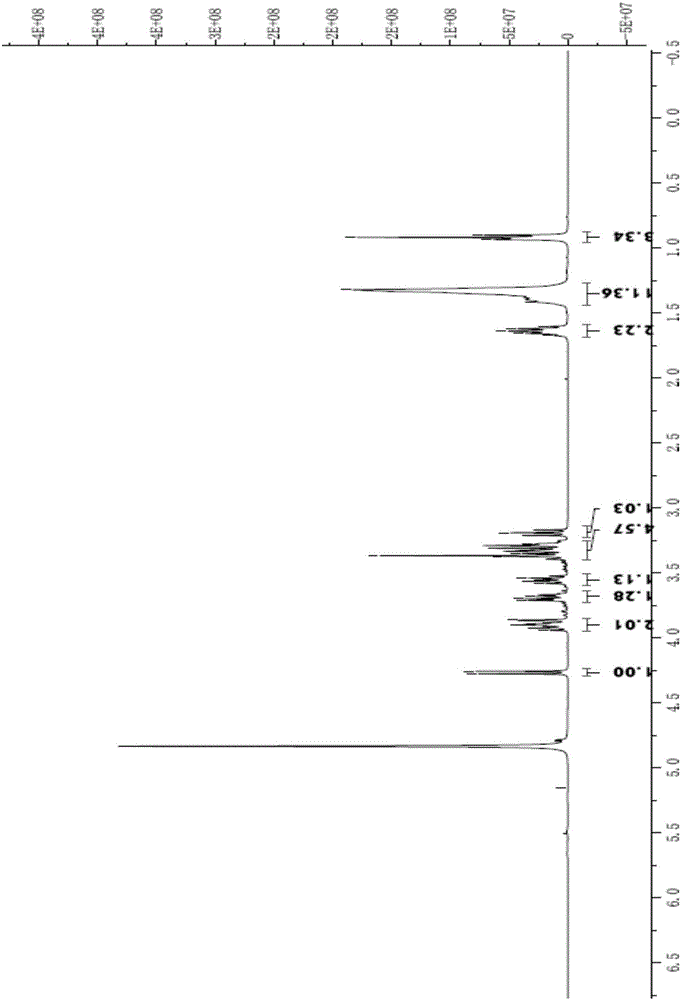
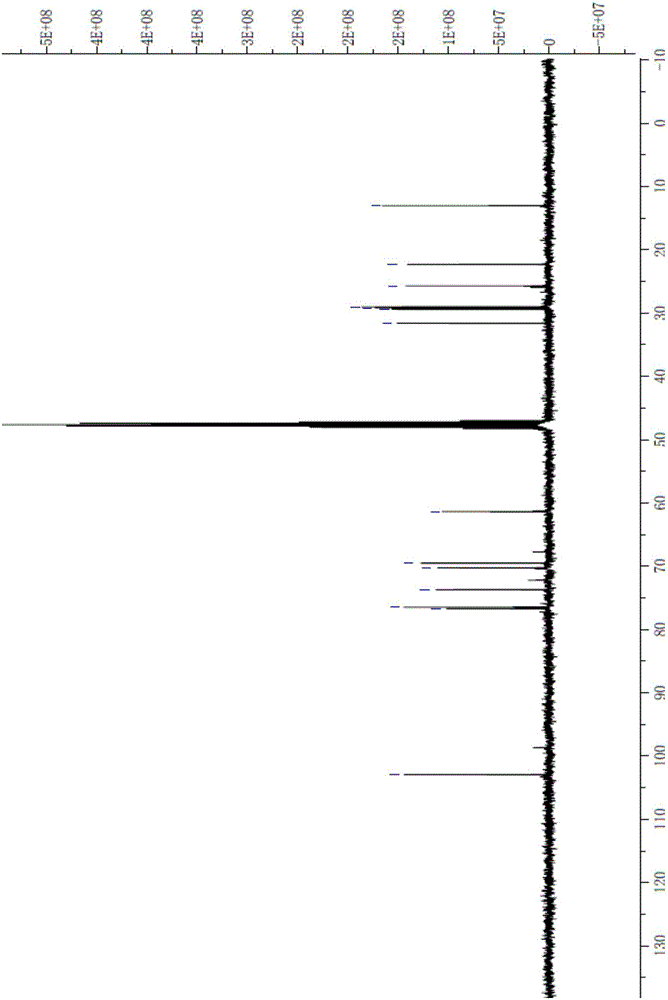
Image
Examples
Embodiment 1
[0025] A kind of synthetic method of n-octyl-β-D-glucopyranoside, comprises the steps:
[0026] 1) Weigh (200g, 0.51mol) peracetylated glucopyranose and dissolve it in 1L of anhydrous dichloromethane, add n-octanol (66.7g, 0.51mol), and add 0.51mol of anhydrous tin tetrachloride, Stir the reaction at room temperature for 30 minutes, wash with 500ml*3 saturated aqueous sodium carbonate solution, collect the organic phase, and distill under reduced pressure to obtain light yellow oily 1-n-octyl-2,3,4,6-tetraacetyl-β-D- Glucopyranoside;
[0027] 2) Dissolve 237 grams of the obtained 1-n-octyl-2,3,4,6-tetraacetyl-β-D-glucopyranoside in 1.2L of methanol, stir at room temperature, add sodium methoxide to adjust the pH to 9, reacted at room temperature for 1.5 hours, adjusted to neutrality with strong acidic cation exchange resin Dowex-509, filtered, and the filtrate was spin-dried to obtain n-octyl-β-D-glucopyranoside (81.9g, 0.28mol) , the yield was 55%, and the β-configuration p...
Embodiment 2
[0030] A synthetic method of n-dodecyl-β-D-glucopyranoside, comprising the steps of:
[0031] 1) Dissolve fully acetylated glucopyranose, n-dodecyl alcohol and anhydrous tin tetrachloride in anhydrous dichloromethane, stir and react at room temperature for 20 minutes, wash with saturated potassium carbonate aqueous solution, collect the organic phase, Distillation under reduced pressure to obtain 1-n-dodecyl-2,3,4,6-tetraacetyl-β-D-glucopyranoside;
[0032] The molar ratio of fully acetylated glucopyranose, n-dodecyl alcohol and anhydrous tin tetrachloride is: 1:1.2:1.2;
[0033] The ratio of fully acetylated glucopyranose to anhydrous dichloromethane is 100g:550ml;
[0034] 2) Dissolve 1-n-dodecyl-2,3,4,6-tetraacetyl-β-D-glucopyranoside in methanol, add sodium methoxide to adjust the pH to 9, and react at room temperature for 1.5 hours, Adjust to neutrality with strong acidic cation exchange resin Dowex-509, filter, distill off the solvent from the filtrate, dry, and detect...
Embodiment 3
[0038] A synthetic method of n-nonyl-β-D-glucopyranoside, comprising the steps of:
[0039] 1) Dissolve fully acetylated glucopyranose, n-nonyl alcohol and anhydrous tin tetrachloride in anhydrous dichloromethane, stir and react at room temperature for 70 minutes, wash with saturated aqueous sodium bicarbonate, collect the organic phase, and reduce Pressure distillation to obtain 1-n-nonyl-2,3,4,6-tetraacetyl-β-D-glucopyranoside;
[0040] The molar ratio of fully acetylated glucopyranose, n-nonyl alcohol and anhydrous tin tetrachloride is: 1:1.1:1;
[0041] The ratio of fully acetylated glucopyranose to anhydrous dichloromethane is 100g:450ml;
[0042] 2) Dissolve 1-n-nonyl-2,3,4,6-tetraacetyl-β-D-glucopyranoside in methanol, add sodium methoxide to adjust the pH to 9, react at room temperature for 1.5 hours, and use strong The acidic cation exchange resin HCRW-20 was adjusted to neutrality, filtered, the filtrate was evaporated to remove the solvent, dried, and detected by ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com