1,2,3-triazole starch derivatives and preparation method thereof
A technology for starch derivatives and triazoles, which is applied in the field of daily chemicals and achieves the effects of high yield, simple synthesis steps, and easy availability of equipment and raw materials
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0033] The synthesis route of 1,2,3-triazole starch is as follows.
[0034]
[0035] Wherein R is an alcoholic hydroxyl group with different chain lengths; the average value range of n is 5-12000.
[0036] In this example, the target compound 1,2,3-triazole starch was synthesized according to the above synthetic route.
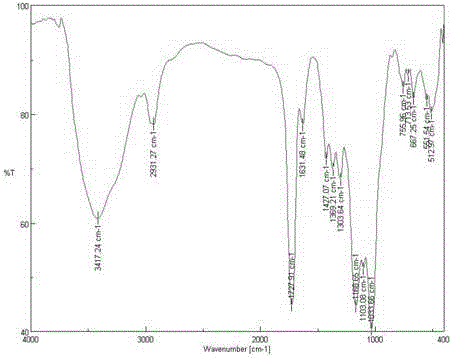
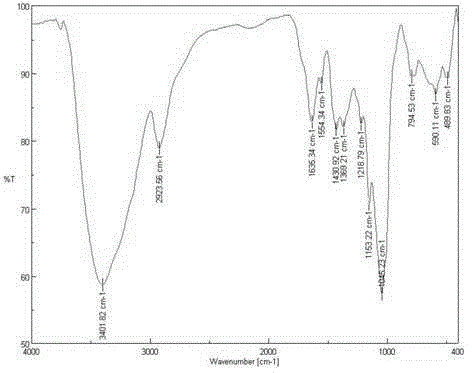
[0037] 1) Preparation of brominated starch: 1.62g starch (see figure 1 ) in 50mL of DMF (N,N-dimethylformamide) at 130°C for 1h, then lower the temperature to 90°C, and add 2.0g of lithium bromide to aid in dissolution. Under ice-cooling, 7.12g of N-bromosuccinimide and 10.49g of triphenylphosphine were added and reacted at 80°C for 3h. Then precipitate with ethanol, wash with ethanol and acetone, and freeze-dry to obtain the product brominated starch (see figure 2 ) 2.01g, set aside.
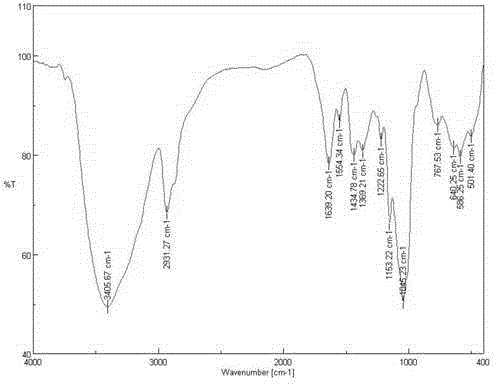
[0038] 2) Preparation of azide starch: 0.225g brominated starch (see figure 2 ) was added to 15mLDMSO (dimethyl sulfoxide), then 0.16g sodium azide was added, and rea...
Embodiment 2
[0041] The difference from Example 1 is:
[0042] 1) Preparation of brominated starch: 1.62g starch (see figure 1 ) in 50mL of DMF (N,N-dimethylformamide) at 120°C for 1h, then cooled down to 80°C, and added 2.0g of lithium bromide to aid in dissolution. Under ice-cooling, add 5.34g of N-bromosuccinimide and 7.87g of triphenylphosphine, and react at 70°C for 3h. Then precipitate with ethanol, wash with ethanol and acetone, and freeze-dry to obtain the product brominated starch (see figure 2 ) 1.84g, set aside.
[0043] 2) Preparation of azide starch: 0.225g brominated starch (see figure 2 ) was added to 10mLDMSO (dimethyl sulfoxide), then 0.13g sodium azide was added, and reacted at 70°C under argon protection for 24h, then directly precipitated with ethanol, washed with ethanol and acetone, and freeze-dried to obtain starch azide (see image 3 ) 0.14g, set aside.
[0044] 3) Preparation of 6-(4-hydroxyethyl)-1,2,3-triazole starch: 0.187g azide starch (see image 3 ) int...
Embodiment 3
[0046] The difference from Example 1 is:
[0047] 1) Preparation of brominated starch: 1.62g starch (see figure 1 ) in 50mL of DMF (N,N-dimethylformamide) at 120°C for 1h, then cooled down to 90°C, and added 2.0g of lithium bromide to aid in dissolution. Under ice-cooling, 7.12g of N-bromosuccinimide and 10.49g of triphenylphosphine were added and reacted at 60°C for 4h. Then precipitate with ethanol, wash with ethanol and acetone, and freeze-dry to obtain the product brominated starch (see figure 2 ) 1.82g, set aside.
[0048] 2) Preparation of azide starch: 0.225g brominated starch (see figure 2 ) was added to 10mLDMSO (dimethyl sulfoxide), then 0.2g sodium azide was added, and reacted at 70°C under argon protection for 24h, then directly precipitated with ethanol, washed with ethanol and acetone, and freeze-dried to obtain starch azide (see image 3 ) 0.17g, set aside.
[0049] 3) Preparation of 6-(4-hydroxypropyl)-1,2,3-triazole starch: 0.187g azide starch (see im...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com