Conductive coating based on noble-metal-loaded polymer nanoparticle and preparation method of conductive coating
A technology of nano-microspheres and conductive coatings, applied in conductive coatings, epoxy resin coatings, coatings, etc., can solve the problems of decreased conductive performance, further improvement of dispersion uniformity and stability, and easy migration.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
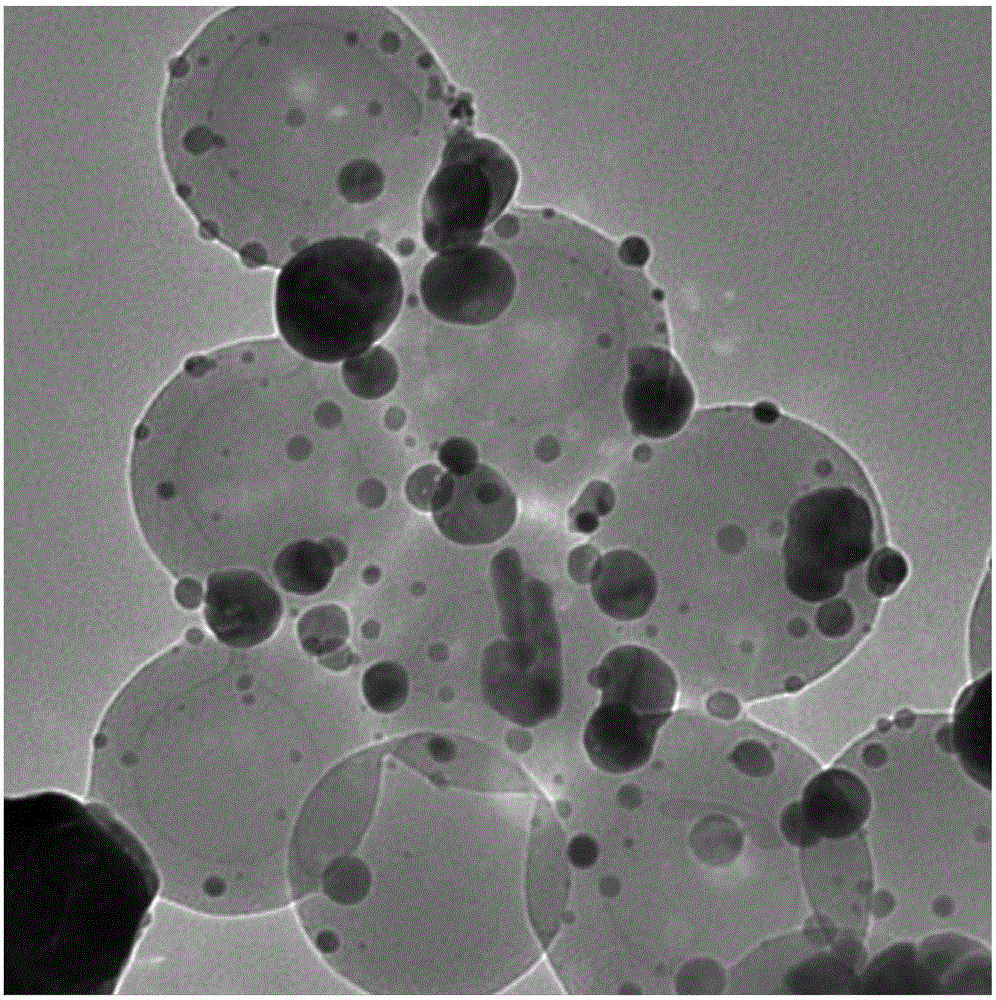
Image
Examples
Embodiment 1
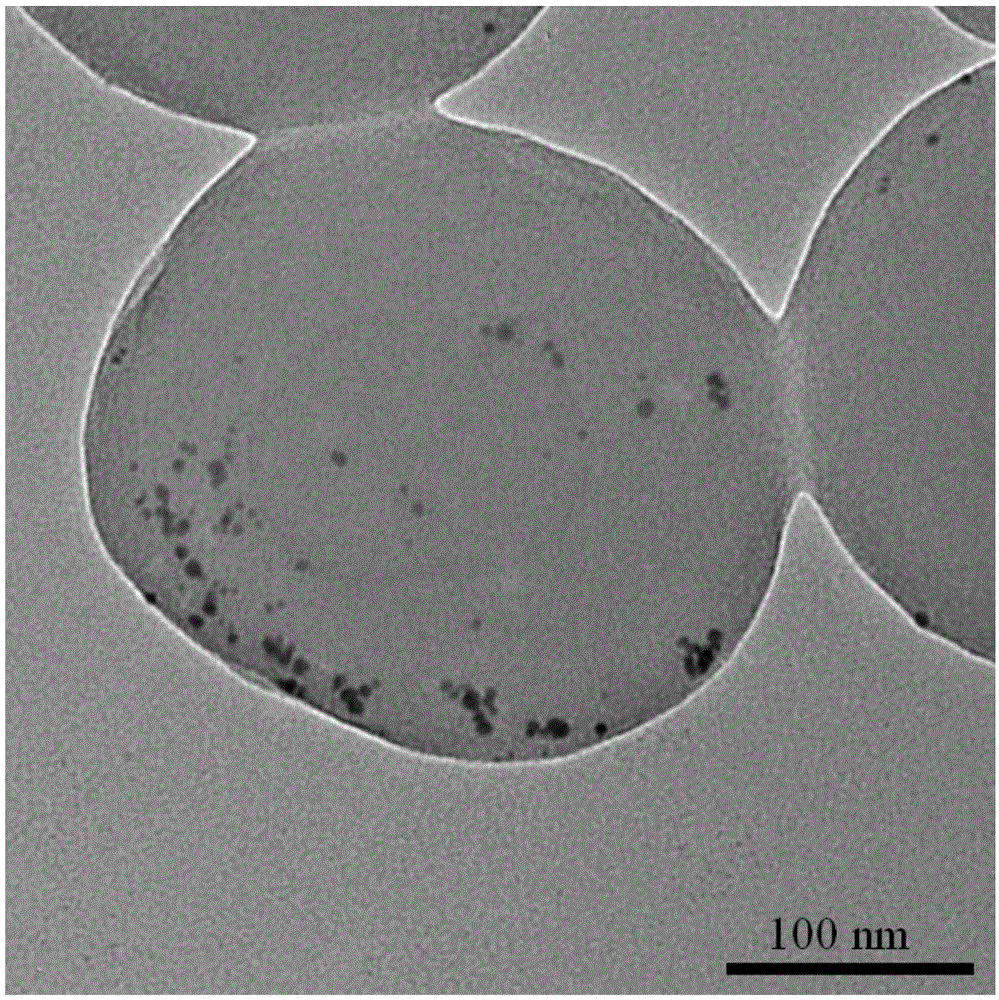
[0029] The preparation of gold-loaded polymer nanospheres is as follows:
[0030] 1) Add 15ml of styrene, 0.5ml of glycidyl methacrylate (GMA), 0.5ml of N,N-dimethylaminoethyl methacrylate (DMAEMA), and 500ml of deionized water into the reaction vessel, under nitrogen protection Stir and heat up to 70°C;
[0031] 2) Add 0.2 g of initiator to the reaction system after purging with nitrogen for 30 minutes, and continue to stir and react for 24 hours under the protection of nitrogen to obtain a polymer nanosphere solution;
[0032] 3) Centrifuge the polymer nanosphere solution obtained in step 2), wash with water and ethanol for 3 to 5 times, and dry in vacuum for 24 hours;
[0033] 4) Disperse 30g of polymer nanospheres in 1L of ethanol, add 50ml of chloroauric acid solution, and dropwise add 50ml of sodium borohydride solution under stirring, react for 12h, wash with ethanol for 3 times, and dry in vacuum for 24h to obtain loaded gold Polymer Nanospheres.
[0034] The conduc...
Embodiment 2
[0038] The preparation of silver-loaded polymer nanospheres is as follows:
[0039] 1) Add 15ml of styrene, 0.7ml of glycidyl methacrylate (GMA), 0.7ml of N,N-dimethylaminoethyl methacrylate (DMAEMA), and 500ml of deionized water into the reaction vessel, under nitrogen protection Stir and heat up to 70°C;
[0040] 2) Add 0.5 g of initiator AIBN to the reaction system after purging with nitrogen for 30 minutes, and continue to stir and react for 24 hours under the protection of nitrogen to obtain a polymer nanosphere solution;
[0041] 3) centrifuging the polymer nanosphere solution obtained in step 2), washing with water and ethanol three times respectively, and drying in vacuum for 24 hours;
[0042] 4) Disperse 40g of polymer nanospheres in 1L of ethanol, add 40ml of silver nitrate solution, and dropwise add 30ml of sodium borohydride solution under stirring, react for 12h, wash with ethanol for 3 times, and dry in vacuum for 24h to obtain silver-loaded polymer nanosphere...
Embodiment 3
[0046] The preparation of gold-loaded polymer nanospheres is as follows:
[0047]1) Add 25ml of styrene, 1ml of glycidyl methacrylate (GMA), 1ml of N,N-dimethylaminoethyl methacrylate (DMAEMA), and 500ml of deionized water into the reaction vessel, stir and Heat up to 70°C;
[0048] 2) Add 1.0 g of initiator AIBN to the reaction system after nitrogen purging for 30 minutes, and continue to stir and react for 24 hours under nitrogen protection to obtain a polymer nanosphere solution;
[0049] 3) centrifuging the polymer nanosphere solution obtained in step 2), washing with water and ethanol three times respectively, and drying in vacuum for 24 hours;
[0050] 4) Disperse 20g of polymer nanospheres in 1L of ethanol, add 30ml of chloroauric acid solution, and dropwise add 30ml of sodium borohydride solution under stirring, react for 12h, wash with ethanol for 3 times, and vacuum dry for 24h to obtain loaded gold Polymer Nanospheres.
[0051] The conductive coating was prepared...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com