Method for producing HIV-1 gp41 recombinant antigen by means of Escherichia coli cell-free system
A hiv-1gp41 and recombinant antigen technology, which is applied in the field of Escherichia coli cell-free system to produce HIV-1gp41 recombinant antigen, can solve the problems of low recovery rate, difficulty in renaturation of inclusion bodies, conformational differences, etc., and achieve improved expression efficiency and good immunity Reactivity, damage avoidance effects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
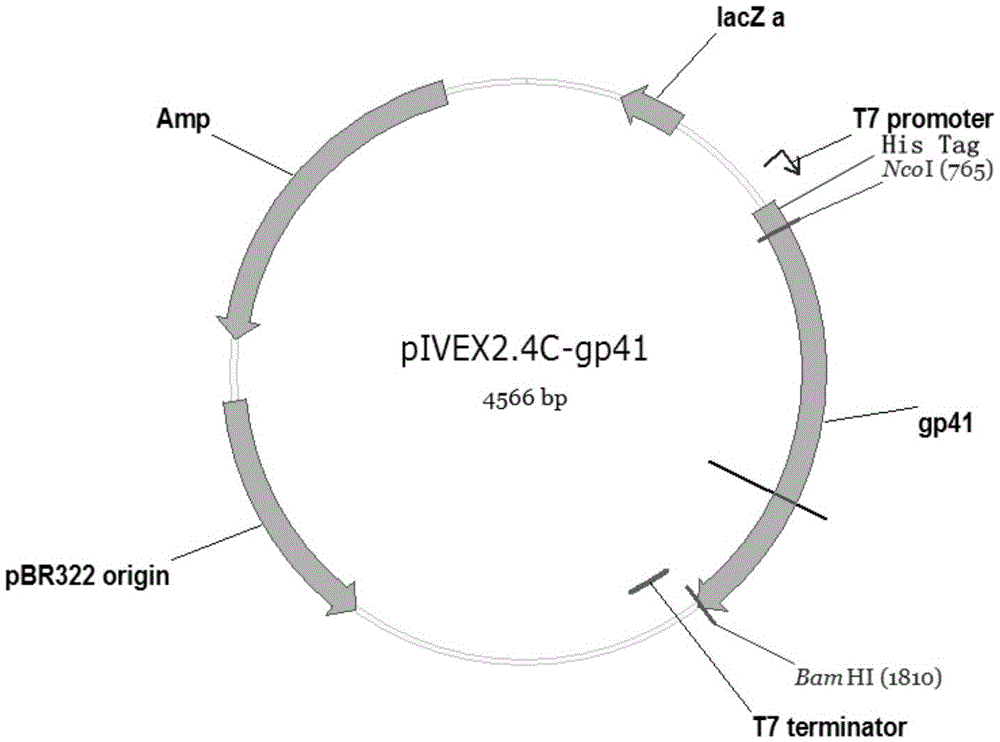
[0026] Embodiment 1, the preparation of recombinant vector pIVEX2.4c-gp41, comprises the following steps:
[0027] 1. Utilize the upstream primer and the downstream primer to amplify the gp41 gene from the synthetic HIV-1 gp41 recombinant antigen gene. The gene sequence of the upstream primer is shown in SEQ ID NO.3, and the gene sequence of the downstream primer is shown in SEQ ID NO.4.
[0028] SEQ ID NO.3:
[0029] CATGCCATGGGCGCTGTTGGTATTGGCGCTCTG
[0030] SEQ ID NO.4:
[0031] CGCGGATCCGCCAGCAGGATGCGCTCCAG
[0032] 2. The PCR reaction conditions are: 94°C pre-denaturation for 5 minutes, 94°C denaturation for 30s, 60°C annealing for 30s, 72°C extension for 60s, in which denaturation, annealing, and extension are repeated 30 times, then 72°C for 10min, and finally Cool down to 4°C and take it out.
[0033] 3. Cell-free expression vectors pIVEX2.4c and gp41 PCR products were digested with NcoI and BamHI at 37°C for 4 hours, respectively, and the digested products recove...
Embodiment 2
[0034] Embodiment 2, the preparation of Escherichia coli cell-free system extract, comprises the following steps:
[0035] 1. Pick a single colony of E.coliBL21(DE3) from the plate and inoculate them in 5mL of LB medium respectively, and cultivate overnight at 37°C with a shaker at 200rpm.
[0036] 2. Transfer all the seed liquid cultivated in step 1 to 500 mL of cell-free fermentation medium.
[0037] 3. Cultivate the shake flask inoculated with E.coliBL21(DE3) at 37°C and 200 rpm until the bacterial concentration OD600 is between 1.5 and 2, then the bacteria can be harvested.
[0038] 4. Pour the bacterial liquid obtained in step 3 into a 500mL centrifuge bottle, centrifuge at 6000g at 4°C for 30min to collect the bacterial cells, discard the supernatant, and obtain the E.coliBL21(DE3) extract according to steps 5-14.
[0039] 5. Resuspend the bacteria with pre-cooled Buffer1 (10mM Tris-acetic acid (pH8.2), 60mM potassium acetate, 14mM magnesium acetate, 1mMDTT, 7mMβ-ME), a...
Embodiment 3
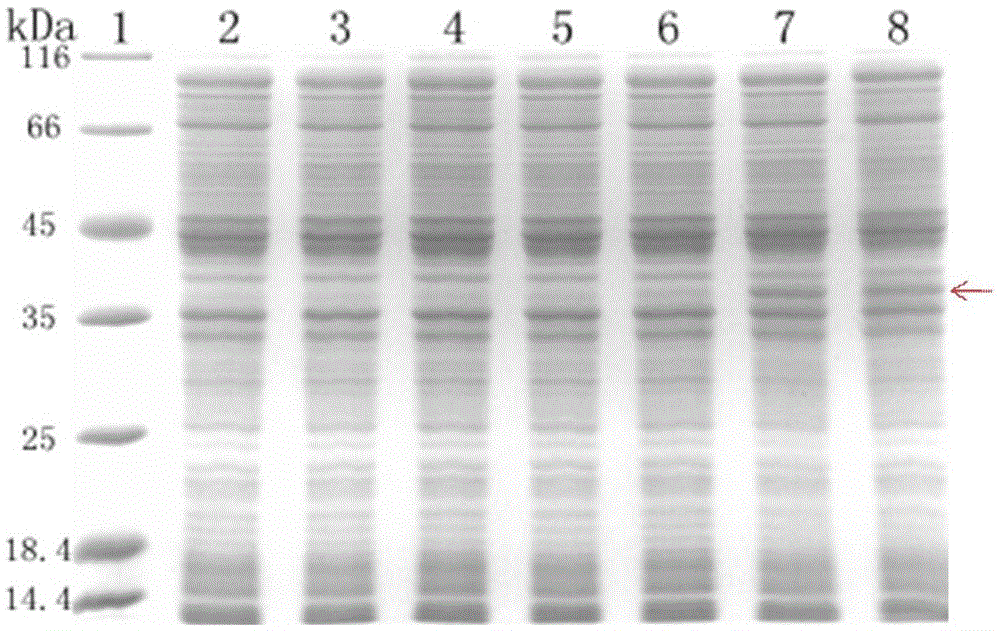
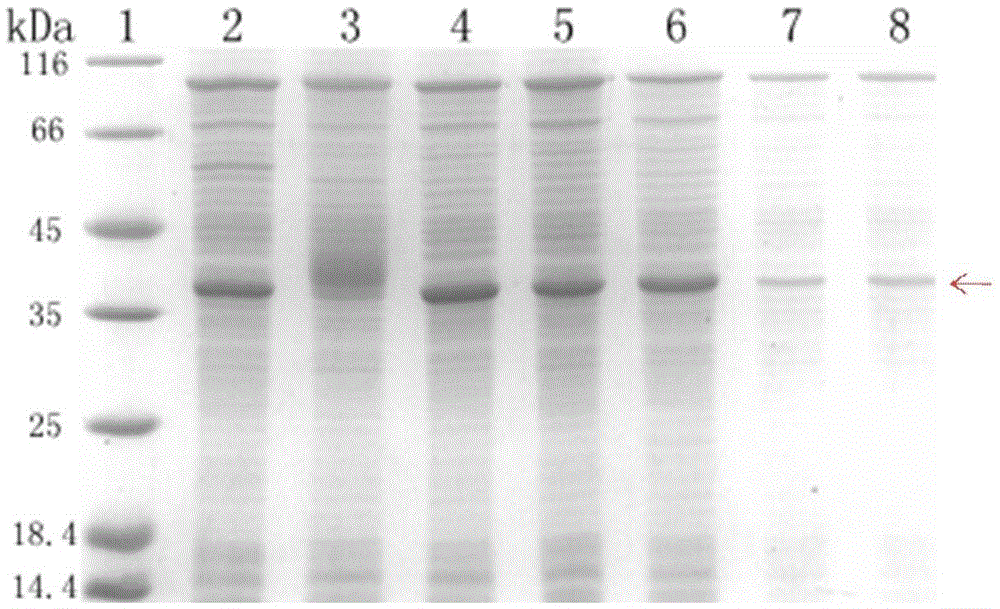
[0049] Embodiment 3, the cell-free system expression of HIV-1gp41 recombinant protein, comprises the following steps:
[0050] 1. The E. coli cell-free expression system was prepared by using the E. coli cell-free system extract prepared in Example 2. The names and amounts of the components in the system are shown in Table 1.
[0051] Table 1: Components of Escherichia coli cell-free expression system
[0052] Components V(μL) E.coli BL21(DE3) extract 24 Energy Mix(EM 3.2×) 16 Amino Acid Mix (25mM) 4
[0053] pIVEX2.4c-gp41 5 water or detergent 1 Total 50
[0054] Wherein, wherein detergent is selected from Tween80 (1%), TritonX-100 (0.2%), β-OG (0.75%), DDM (0.1%), Brij78 (0.5%), Brij58 (0.5%) (mass volume Fraction g / ml). The preparation method of EnergyMix (3.2×) is shown in Table 2.
[0055] Table 2 EnergyMix (3.2×) ingredient list
[0056] Components Stock Final conc. Volume(μL) DTT 10...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com