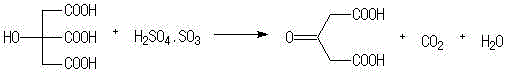Synthetic method of high-quality acetonedicarboxylic acid and acetonedicarboxylate
A technology of acetone dicarboxylate and acetone dicarboxylate, which is applied in the field of synthesis of high-quality acetone dicarboxylate and acetone dicarboxylate, to achieve the effects of light equipment corrosion, low environmental pressure, and easy purification
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
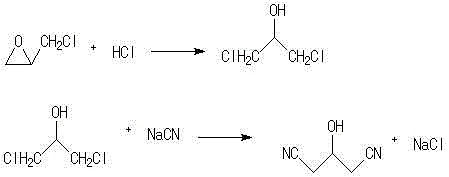
[0010] Example 1. In a 250ml three-neck flask, add 50g (0.5mol) of concentrated hydrochloric acid (chemically pure, content 36.5%), stir well, raise the temperature to 30°C, add 42.9g (0.436mol) of epichlorohydrin dropwise, The dropwise addition was completed in about 45 minutes, and the reaction was incubated for another 2 hours. After the reaction was completed, the mixture was left to stand for separation, and the organic phase was separated into 1,3-dichloropropanol, which was dehydrated with anhydrous sodium carbonate at room temperature.
[0011] In a 250ml three-necked flask, add 138.8 grams of 30% sodium cyanide aqueous solution (0.85mol), raise the temperature to reflux, slowly add the organic phase of the step reaction dropwise, and the dropwise addition is completed in about 1 hour, and keep the temperature for 2 hours, cool to room temperature, and divide The organic phase is 1,3-dinitrile propanol, and washed with 30ml*3 water.
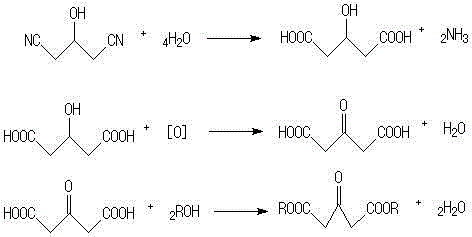
[0012] In a 250ml three-necked fl...
Embodiment 2
[0015] Example 2. In a 250ml three-necked flask, add hydrobromic acid and 50 grams (0.5mol) of concentrated hydrochloric acid (chemically pure, content 36.5%), and stir well, raise the temperature to 30°C, and dropwise add 42.9 grams of epichlorohydrin ( 0.436mol), the dropwise addition was completed in about 45 minutes, and then incubated for 2 hours. After the reaction was completed, the mixture was left to stand for separation, and the organic phase was separated into 1,3-dichloropropanol, which was dehydrated with anhydrous sodium carbonate at room temperature.
[0016] In a 250ml three-necked flask, add 138.8 grams of 30% sodium cyanide aqueous solution (0.85mol), raise the temperature to reflux, slowly add the organic phase of the step reaction dropwise, and the dropwise addition is completed in about 1 hour, and keep the temperature for 2 hours, cool to room temperature, and divide The organic phase is 1,3-dinitrile propanol, and washed with 30ml*3 water.
[0017] In a...
Embodiment 3
[0020] Example 3. In a 250ml three-necked flask, add hydrofluoric acid and 50 grams (0.5mol) of concentrated hydrochloric acid (chemically pure, content 36.5%), and stir well, raise the temperature to 30°C, and dropwise add 42.9 grams of epichlorohydrin ( 0.436mol), the dropwise addition was completed in about 45 minutes, and then incubated for 2 hours. After the reaction was completed, the mixture was left to stand for separation, and the organic phase was separated into 1,3-dichloropropanol, which was dehydrated with anhydrous sodium carbonate at room temperature.
[0021] In a 250ml three-necked flask, add 138.8 grams of 30% sodium cyanide aqueous solution (0.85mol), raise the temperature to reflux, slowly add the organic phase of the step reaction dropwise, and the dropwise addition is completed in about 1 hour, and keep the temperature for 2 hours, cool to room temperature, and divide The organic phase is 1,3-dinitrile propanol, and washed with 30ml*3 water.
[0022] In ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com