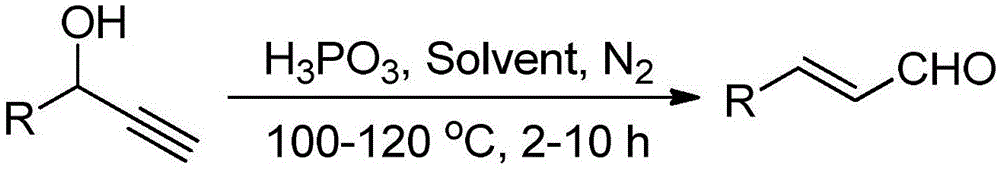
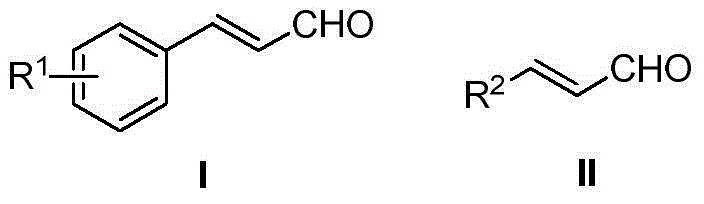
Method for preparing cinnamyl aldehyde compounds
A compound, the technology of cinnamaldehyde, which is applied in the field of preparation of cinnamaldehyde compounds, can solve the problems of pretreatment and preparation of catalysts, and achieve the effects of easy separation, high yield, and cheap and easy-to-obtain raw materials
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Synthetic example 1
[0021] Synthesis of Cinnamaldehyde
[0022] Add 0.2mmol 1-phenyl-2-propyn-1-ol, 0.30mmol 50wt% phosphorous acid aqueous solution, and 0.5mL dichloromethane into the reactor. Under nitrogen atmosphere, heat to 110°C, keep stirring for 2h, stop the reaction, cool to room temperature, add saturated sodium carbonate solution to wash, extract with dichloromethane, dry, distill off the solvent under reduced pressure, and separate the crude product by column chromatography to obtain Target product, yield 91%. 1 HNMR (400MHz, CDCl 3 ):δ9.70(d,J=7.7Hz,1H),7.57-7.55(m,2H),7.49-7.42(m,4H),6.72(dd,J=16.0,7.7Hz,1H). 13 CNMR (100MHz, CDCl 3 ): δ193.6, 152.7, 133.9, 131.2, 129.0, 128.5, 128.4.
Synthetic example 2
[0024] Synthesis of p-fluorocinnamaldehyde
[0025] Add 0.2mmol of 1-p-fluorophenyl-2-propyn-1-ol, 0.30mmol of 50wt% phosphorous acid aqueous solution, and 0.5mL of dichloromethane into the reactor. Under nitrogen atmosphere, heat to 110°C, keep stirring for 2h, stop the reaction, cool to room temperature, add saturated sodium carbonate solution to wash, extract with dichloromethane, dry, distill off the solvent under reduced pressure, and separate the crude product by column chromatography to obtain Target product, yield 88%. 1 HNMR (400MHz, CDCl 3 ): δ9.69(d, J=7.6Hz, 1H), 7.59-7.54(m, 2H), 7.44(d, J=16.0Hz, 1H), 7.15-7.09(m, 2H), 6.65(dd, J=16.0,7.6Hz,1H). 13 CNMR (100MHz, CDCl 3 ):δ193.4,165.42(d,J C-F =253.2Hz), 151.3, 130.4(d, J C-F =8.7Hz), 130.2(d, J C-F =3.4Hz), 128.3(d, J C-F =2.3Hz), 116.3(d, J C-F =22.1Hz).
Synthetic example 3
[0027] Synthesis of p-chlorocinnamaldehyde
[0028]Add 0.2mmol of 1-p-chlorophenyl-2-propyn-1-ol, 0.30mmol of 50wt% phosphorous acid aqueous solution, and 0.5mL of dichloromethane into the reactor. Under nitrogen atmosphere, heat to 110°C, keep stirring for 2h, stop the reaction, cool to room temperature, add saturated sodium carbonate solution to wash, extract with dichloromethane, dry, distill off the solvent under reduced pressure, and separate the crude product by column chromatography to obtain Target product, yield 85%. 1 HNMR (400MHz, CDCl 3 ):δ9.70(d,J=7.6Hz,1H),7.50(d,J=8.5Hz,2H),7.45-7.40(m,3H),6.69(dd,J=16.0,7.6Hz,1H) . 13 CNMR (100MHz, CDCl 3 ): δ193.3, 151.0, 137.2, 132.4, 129.6, 129.4, 128.9.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com