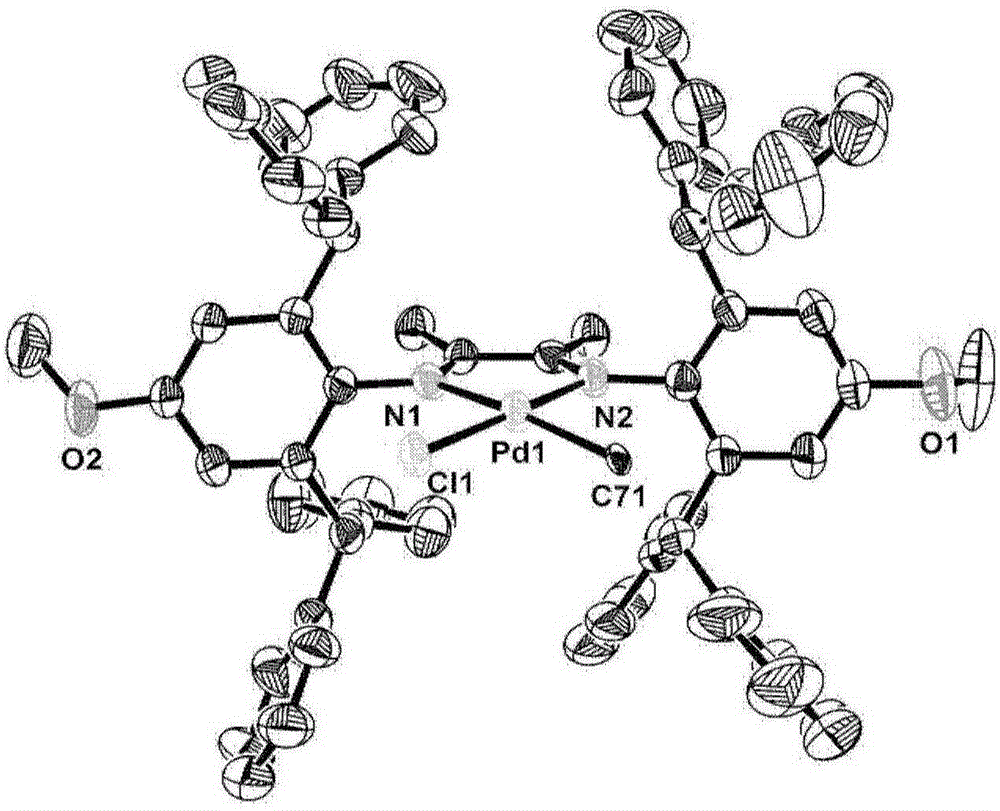
Diimine palladium catalyst with great steric hindrance, and ligand, preparation method and application thereof
A compound and complex technology, applied in the field of large sterically hindered diimide palladium catalysts and their ligands, can solve the problems of low molecular weight, limited application, low activity, etc., and achieve the effect of high thermal stability and activity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0070] Example 1: Synthesis of N,N-bis(2,6-diphenylmethyl-4-methoxyphenyl)butane-2,3-diimine
[0071]
[0072] At room temperature, add 9.11 g of 2,6-benzyl-4-methoxyaniline and 0.86 of diacetyl into an eggplant-shaped bottle (equipped with a thermometer, reflux condenser and stirrer) containing 200 ml of toluene. Gram and 20 mg of p-toluenesulfonic acid were heated to 80°C in an oil bath to react for 24 hours. Then, use a water separator (Xinville 14-port water separator) to divide the water and reflux for three days. Use petroleum ether and dichloromethane 1:1 to spot the board, and you will find that there is mainly one point, indicating that the reaction has ended. Finally, part of the solvent was removed by rotary evaporation, and then 300 ml of methanol was added to the remaining reaction solution to precipitate a large amount of yellow solid. The solid was obtained by filtration under reduced pressure, washed three times with 20 ml of methanol, and dried under vacuum to o...
Embodiment 2
[0074] Example 2: Synthesis of N,N-bis(2,6-diphenylmethyl-4-chlorophenyl)butane-2,3-diimine
[0075]
[0076] Similar to Example 1, at room temperature, add 9.20 g of 2,6-benzyl-4-chloroaniline, 0.86 g of butanedione and p-toluenesulfonic acid into an eggplant-shaped bottle containing 200 ml of toluene 20 mg, and heated to 80°C in an oil bath to react for 24 hours, and then use a water separator (Xinville 14 ports) to divide the water and reflux for seven days. Use petroleum ether and dichloromethane 1:1 to point the board, you will find that it is mainly a point. Indicates that the reaction has ended. Part of the solvent was removed by rotary evaporation, and then 300 ml of methanol was added to the remaining reaction solution, and a large amount of yellow solid was precipitated. The solid was obtained by filtration under reduced pressure with a water pump, washed three times with 20 ml of methanol, and dried under vacuum to obtain 8.82 g of solid with a yield of 91%.
[0077] 1...
Embodiment 3
[0078] Example 3: Synthesis of N,N-bis(2,6-diphenylmethyl-4-trifluoromethylphenyl)butane-2,3-diimine
[0079]
[0080] Similar to Example 1, at room temperature, add 9.87 g of 2,6-benzyl-4-trifluoromethylaniline, 0.86 g of butanedione and p-methyl to an eggplant-shaped bottle containing 200 ml of toluene 20 mg of benzene sulfonic acid, heated to 80°C in an oil bath for 24 hours, and then used a water separator (Xinville 14) to reflux the water for seven days. Use petroleum ether and dichloromethane 1:1 to spot the board and you will find that it is mainly one Click to indicate that the reaction has ended. Part of the solvent was removed by rotary evaporation, and then 300 ml of methanol was added to the remaining reaction solution, and a large amount of yellow solid was precipitated. The solid was obtained by filtration under reduced pressure, washed three times with 20 ml of methanol, and dried under vacuum to obtain 9.03 g of solid with a yield of 90%.
[0081] 1 HNMR(CDCl 3 , ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| melting point | aaaaa | aaaaa |
| melting point | aaaaa | aaaaa |
| melting point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com