Method for efficiently producing 1, 3-propylene glycol by reducing by-product acetic acid
A propylene glycol, high-efficiency technology, applied in the field of bioengineering, can solve problems such as no detailed research and no reports, and achieve the effect of fast product synthesis, less by-products, and high conversion rate
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
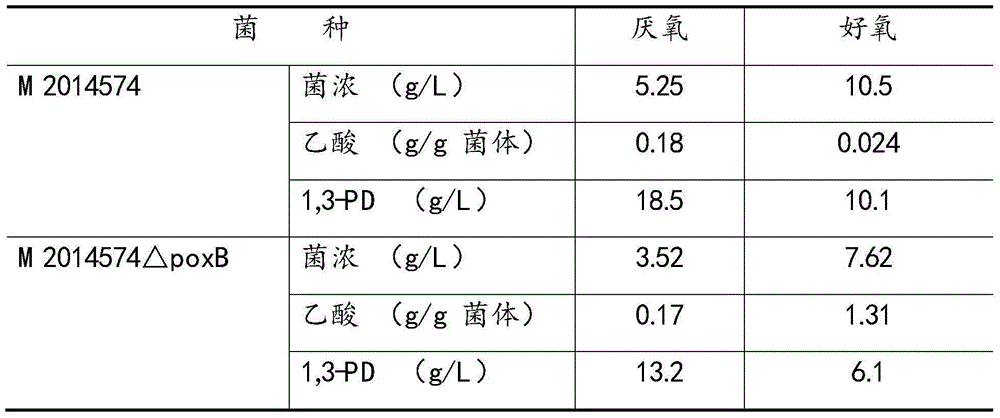
[0022] Example 1. Knocking out the poxB gene alone severely reduces the growth of bacteria, which is not conducive to the synthesis of 1,3-PD
[0023] The M2014574 strain was cultured overnight at 37° C. in LB medium (0.5% yeast extract, 1% tryptone, 1% NaCl, pH 7.0), and the genome was extracted. Using the extracted M2014574 genome as a template, primers were designed based on the poxB gene (locus_tag: KPN_00904) on the genome sequence of Klebsiella pneumoniae MGH78578 (LOCUS: NC_009648) registered by NCBI, and the target band was recovered after PCR reaction And sequenced, the target band was subjected to gene analysis after sequencing, and the similarity with the poxB gene on MGH78578 was 100%. The poxB gene (1278bp) of the M2014574 genome was knocked out by means of homologous recombination, and the obtained recombinant strain was M2014574ΔpoxB.
[0024] M2014574 and M2014574△pta were inoculated in 250ml Erlenmeyer flasks respectively for anaerobic and aerobic culture at ...
Embodiment 2
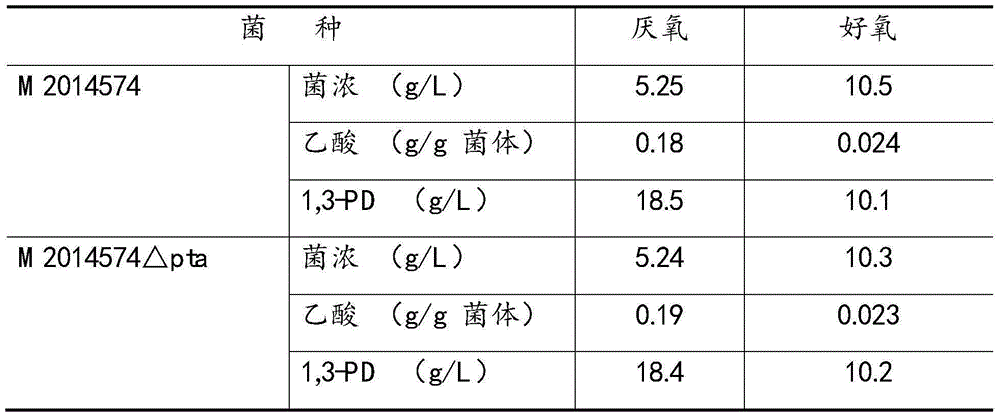
[0028] Example 2. Knocking out the pta gene alone has almost no effect on the growth of the bacteria and the synthesis of 1,3-PD
[0029] The M2014574 strain was cultured overnight at 37° C. in LB medium (0.5% yeast extract, 1% tryptone, 1% NaCl, pH 7.0), and the genome was extracted. Using the extracted M2014574 genome as a template, primers were designed based on the pta gene (locus_tag: KPN_02688) on the genome sequence of Klebsiella pneumoniae MGH78578 registered in NCBI (LOCUS: NC_009648), and the target band was recovered after PCR reaction And sequenced, the target band was subjected to gene analysis after sequencing, and the similarity with the poxB gene on MGH78578 was 100%. The poxB gene (2133bp) of the M2014574 genome was knocked out by means of homologous recombination, and the obtained recombinant strain was M2014574Δpta.
[0030] M2014574 and M2014574△pta were inoculated in 250ml Erlenmeyer flasks respectively for anaerobic and aerobic culture at 37°C for 12 hours...
Embodiment 3
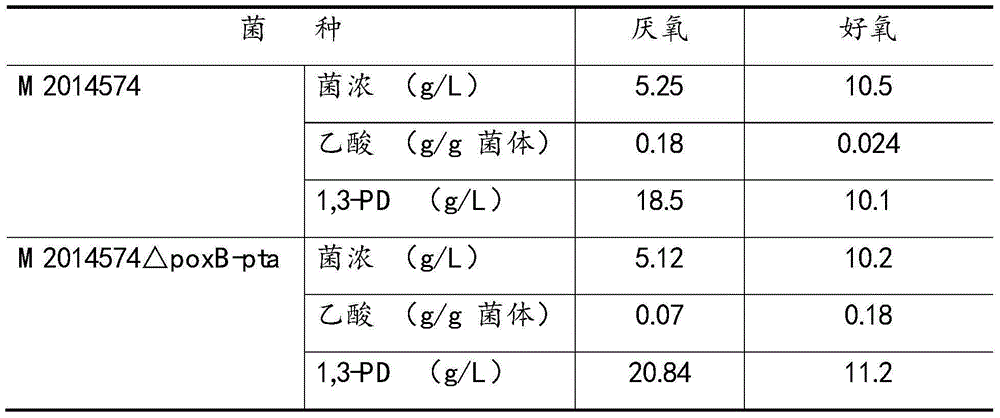
[0034] Example 3. Knocking out the poxB and pta genes at the same time did not significantly inhibit the bacteria, but the synthesis of 1,3-PD was improved
[0035] Klebsiella pneumoniae M2014574 was simultaneously knocked out according to the methods in Examples 1 and 2, and the obtained recombinant bacteria were M2014574ΔpoxB-pta.
[0036] M2014574 and M2014574△poxB-pta were respectively inoculated in 250ml Erlenmeyer flasks for anaerobic and aerobic culture at 37°C for 12 hours, and the medium was seed medium supplemented with 40g / L glycerol. During aerobic culture, the flask was sealed with 8 layers of gauze; under anaerobic culture conditions, the air in the flask before inoculation was replaced with nitrogen, and the flask was sealed with a rubber stopper.
[0037] After culturing for 12 hours, the bacterial cell concentration, 1,3-PD and acetic acid concentrations in the fermentation broth were measured, and the results are shown in Table 3. It can be seen from Table 2...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com