Synthetic method for high-purity iron phosphate and doped metallic element thereof
A technology of high-purity iron phosphate and metal elements, which is applied in the direction of electrical components, active material electrodes, battery electrodes, etc., can solve the problems of hindering lithium extraction/intercalation, lithium content reduction, environmental pollution, etc., and reduce the cost of reaction equipment and control Requirements, reduce waste water discharge, reduce the effect of production cost
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0028] (1) With ferrous oxalate (purity 99%) as iron source, phosphoric acid (purity 85%) as phosphorus source, according to molar ratio: n(Fe):n(P)=1:1.01 ratio, weigh oxalate Iron 87.80g, phosphoric acid 69.86g, polyethylene glycol is 0.02wt% of iron source;
[0029] (2) Dissolve phosphoric acid in 2L of distilled water, transfer it into a 3L reaction kettle, add polyethylene glycol, stir at 60rpm for 5min, and heat to 60°C in a water bath;
[0030] (3) Slowly add ferrous oxalate, and adjust the stirring speed to 300rpm;
[0031] (4) react for 3h until the precipitate turns white;
[0032] (5) Aging for 1 hour after the completion of the reaction, washing with distilled water until the pH value = 6.5, filtering and drying the precipitate at 120°C for 4 hours to obtain white ferric phosphate hydrate (FePO 4 2H 2 O).
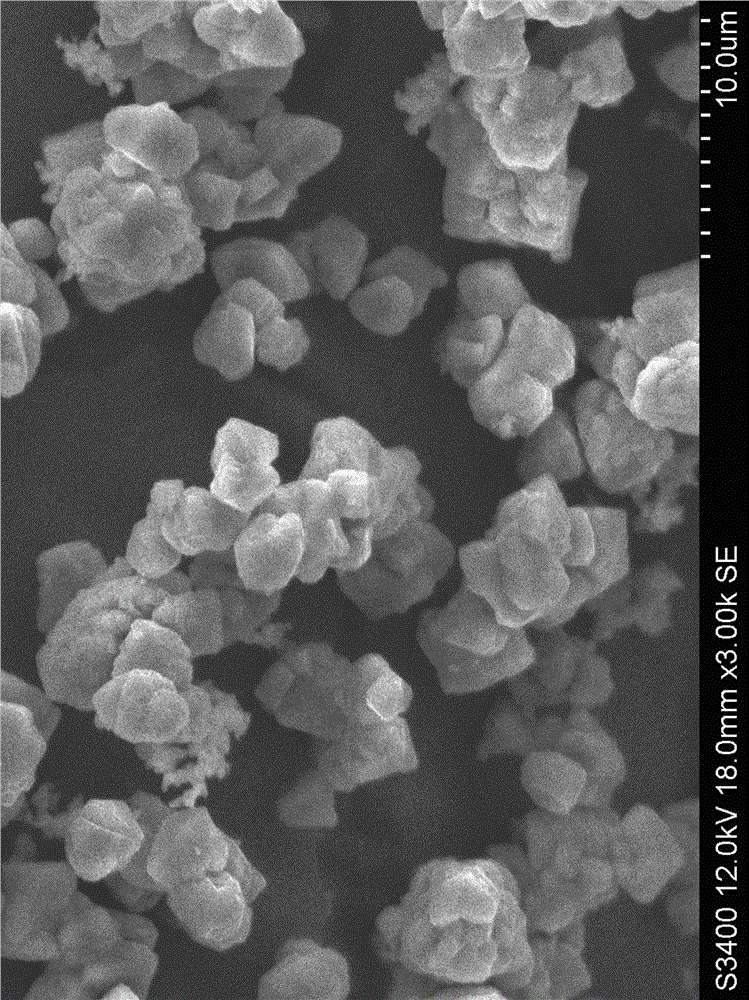
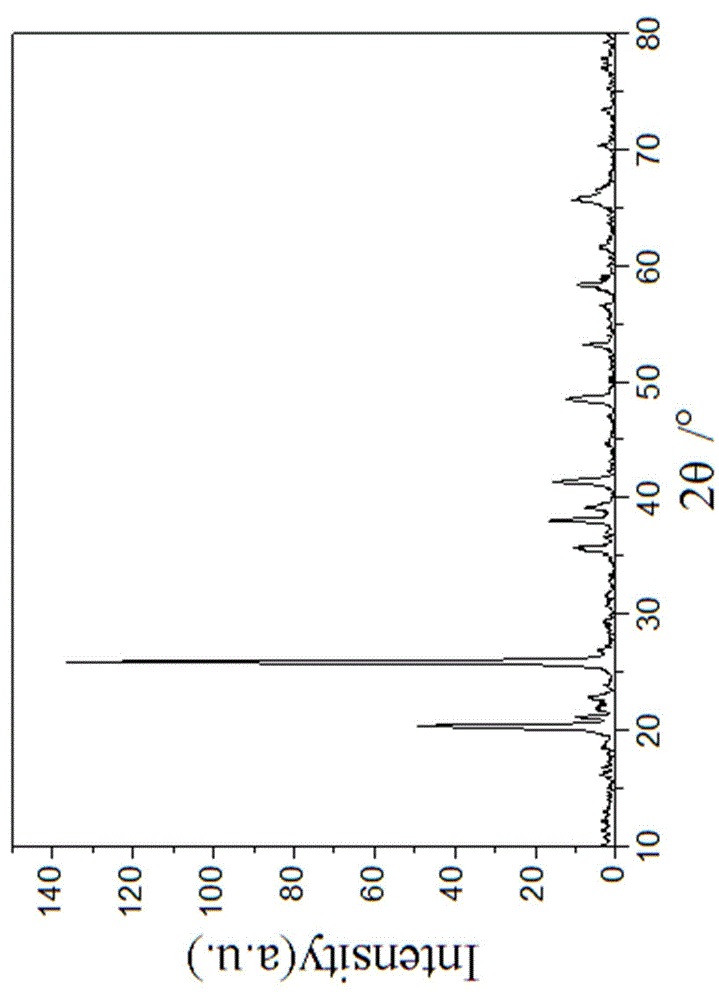
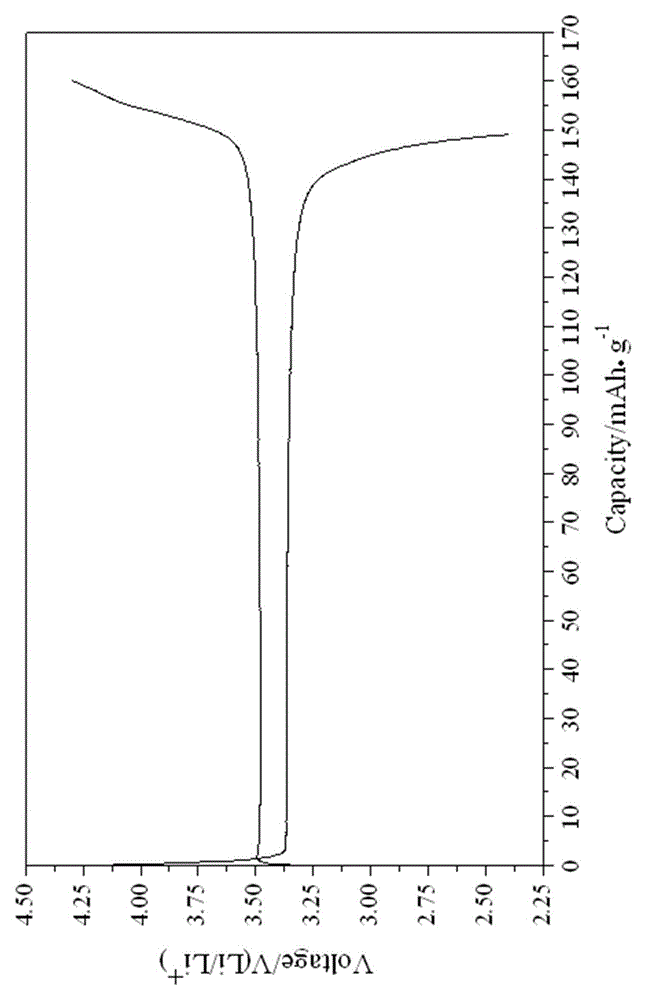
[0033] The particle size of the obtained water and iron phosphate is 2~5μm (such as figure 1 shown), with a purity of 99.5%; the synthesized FePO 4 2H 2 ...
Embodiment 2
[0035] (1) With ferric oxide (purity 99%) as iron source, phosphoric acid (purity 85%) as phosphorus source, according to molar ratio: n(Fe):n(P)=1:1.02 ratio, weigh three Ferric oxide 48.73g, phosphoric acid 70.55g, polyvinylpyrrolidone is 0.05wt% of iron source;
[0036] (2) Dissolve phosphoric acid in 2L of distilled water, transfer it into a 3L reaction kettle, add polyvinylpyrrolidone, stir at 60rpm for 5min, and heat to 80°C in a water bath;
[0037] (3) Slowly add ferrous oxalate, and adjust the stirring speed to 1000rpm;
[0038] (4) After 2 hours of reaction, when the solution is gray-green, add 20ml H 2 o 2 , the precipitate turned yellow-white, and continued to stir for 2 hours;
[0039] (5) Aging for 2 hours after the completion of the reaction, then washing with distilled water until the pH value = 6.5, filtering and drying the precipitate at 110°C for 5 hours to obtain yellow-white ferric phosphate hydrate (FePO 4 2H 2 (2), the purity is 99.5%;
[0040] Usi...
Embodiment 3
[0042] (1) Using ferrous oxalate (purity 99%) as iron source, phosphoric acid (purity 85%) as phosphorus source, prepared by doping manganese dioxide Molar ratio: n(Fe):n(P):n(Mn )=0.97:1.01:0.03 ratio, take ferrous oxalate 85.16g, phosphoric acid 70.55g, manganese dioxide 1.60g, polyvinylpyrrolidone is 0.03wt% of iron source;
[0043] (2) Fully mix manganese dioxide and ferrous oxalate with a ball mill at a speed of 200 rpm for 1 hour;
[0044] (3) Dissolve phosphoric acid in 2.5L of distilled water and move it into a 4L reaction kettle, add polyvinylpyrrolidone, stir at 60rpm for 5min, heat in a water bath to 60°C; slowly add ferrous oxalate and dioxide A mixture of manganese, and adjust the stirring speed to 1500rpm;
[0045] (4) After reacting for 2 hours, add 20ml H 2 o 2 , the precipitate turned yellow-white, and the stirring was continued for 6 hours;
[0046] (5) Aging for 6 hours after the completion of the reaction, then washing with distilled water to pH = 7.0, ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com