Method for preparing polylactone
A technology of polylactone and caprolactone is applied in the fields of organic catalysis and polymer materials, which can solve the problems of unsatisfactory ring-opening polymerization effect, high catalyst price and high catalyst conditions, and achieves enhanced process feasibility and high reaction selectivity. , the effect of fast aggregation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
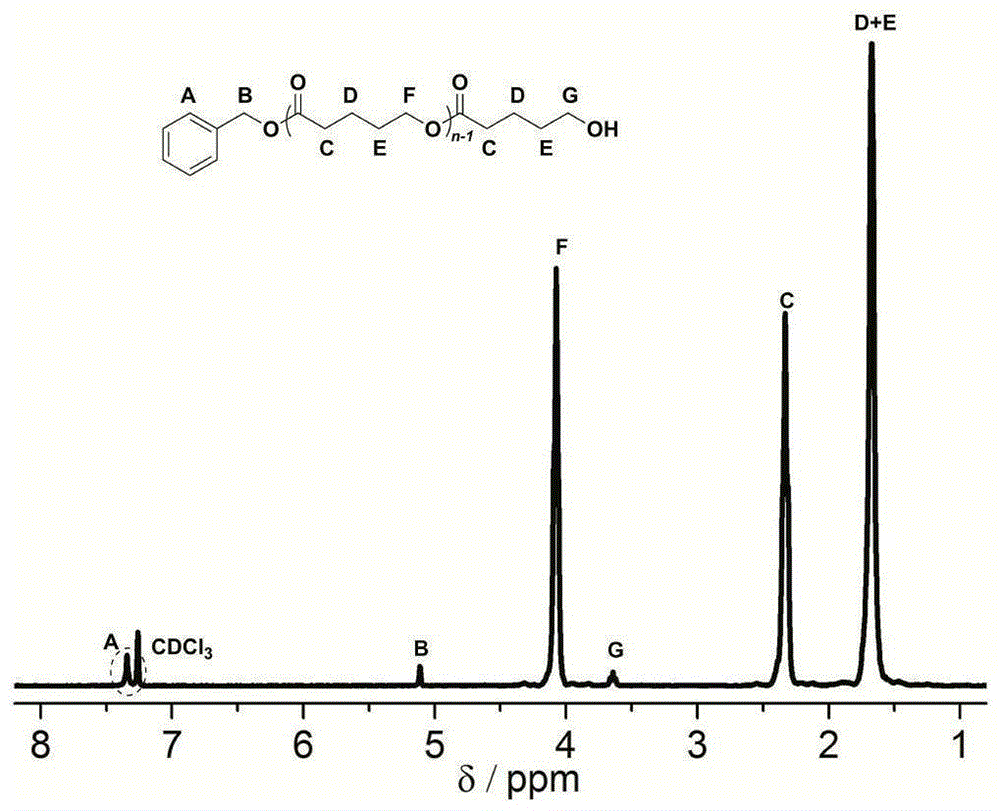
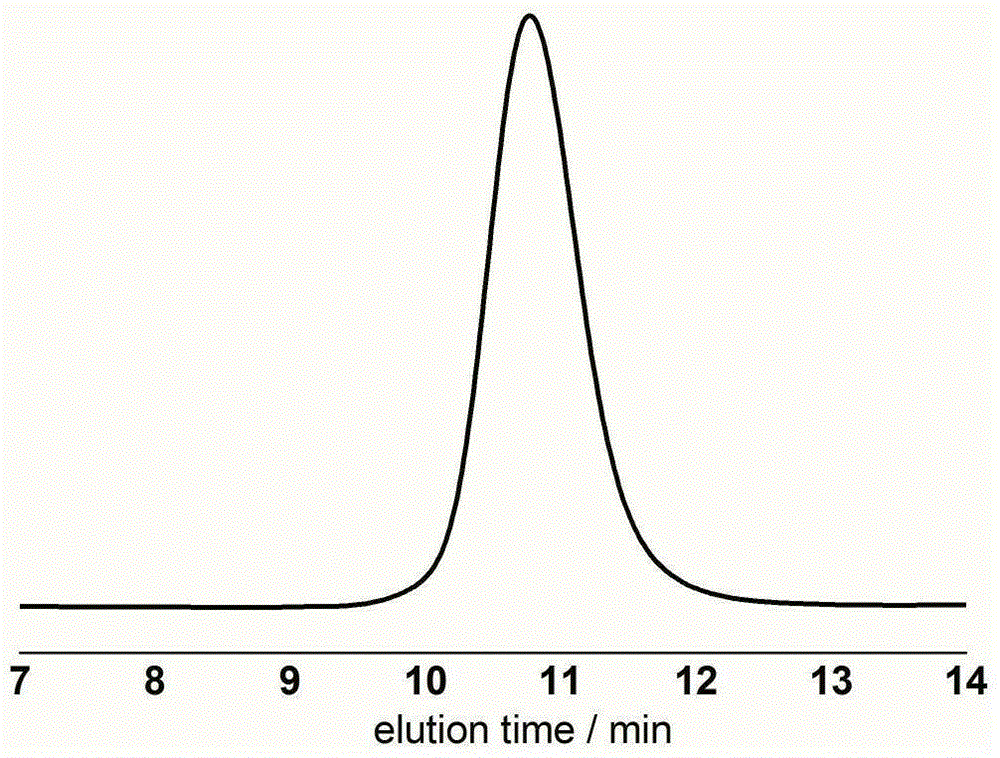
[0028] γ-Resorcinol (0.0154g, 0.1mmol, 1.0equiv), δ-valerolactone (0.27ml, 3.0mmol, 30equiv) and benzyl alcohol (10.3μL, 0.1mmol, 1.0equiv) were added to the reaction flask, Dissolved with 1.0ml of dichloromethane, under the protection of argon, stirred and reacted at room temperature for 18 hours, concentrated the reactant and poured it into methanol, filtered the precipitate and dried to constant weight to obtain a white polyvalerolactone product, the conversion rate It is 94% (proton nuclear magnetic resonance spectrum, 400MHz, CDCl 3 ), the number average molecular weight M of polyvalerolactone n 3090gmol -1 (Proton NMR spectrum, 400MHz, CDCl 3 ), the dispersion PDI is 1.08 (molecular exclusion chromatography, Waterscolumn: 5mm, 300 × 7.8mm, tetrahydrofuran mobile phase, 0.7mLmin -1 , polystyrene as standard), 1 HNMR (300MHz, CDCl 3 ):δ(ppm)1.68(m,2H×n,(–CH 2 CH 2 CH 2 O–) n ),1.70(m,2H×n,(–COCH 2 –CH 2 CH 2 –) n ),2.34(t,2H×n,J=6.8Hz,(–OCOCH 2 CH 2 –) n ),...
Embodiment 2
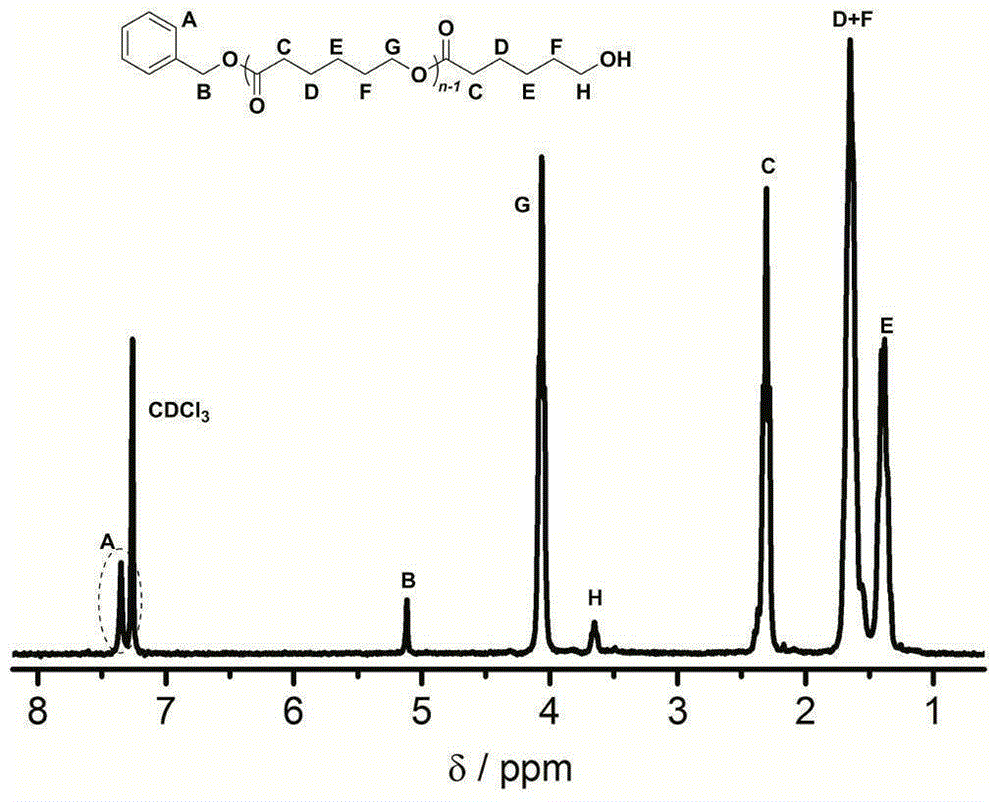
[0030]γ-Resorcinol (0.0154g, 0.1mmol, 1.0equiv), ε-caprolactone (0.33ml, 3.0mmol, 30equiv) and propynyl alcohol (5.8μL, 0.1mmol, 1.0equiv) were added to the reaction flask , dissolved with 1.0ml of toluene, under the protection of argon, stirred and reacted at room temperature for 24 hours, the reactant was concentrated and poured into ethanol, the precipitate was filtered and dried to constant weight to obtain a white polyvalerolactone product, and the conversion rate was 93%, the number average molecular weight of polyvalerolactone M n 4320gmol -1 , the dispersion PDI is 1.06, 1 HNMR (300MHz, CDCl 3 ):δ(ppm),1.39(m,2H×n,(–CH 2 CH 2 CH 2 CH 2 CH 2 –) n ),1.63(m,2H×n,(–CH 2 CH 2 CH 2 O–) n ),1.68(m,2H×n,(–COCH 2 CH 2 CH 2 –) n ),2.31(t,2H×n,J=7.3Hz,(–OCOCH 2 CH 2 –) n ),3.65(t,2H,J=6.6Hz,CH 2 CH 2 OH), 4.06(t, 2H×n, J=6.6Hz, (–CH 2 CH 2 O–) n ),5.12(s,2H,ArCH 2 O), 7.23–7.39 (m, 5H, aromatic); polyε-caprolactone 1 HNMR spectrum see figure 2 .
Embodiment 3
[0032] γ-Resorcinol (0.0154g, 0.1mmol, 1.0equiv), butyl caprolactone (3.3ml, 30.0mmol, 300equiv) and N-(2-hydroxyethyl)maleimide (14.112g , 0.1mmol, 1.0equiv) into the reaction flask, dissolved with 2.0ml of tetrahydrofuran, under the protection of argon, stirred at room temperature for 48 hours, then concentrated the reactant and poured it into ether, precipitated and filtered and dried to constant weight, Obtain white polyvalerolactone product, conversion rate is 82%, the number average molecular weight M of polybutyl position caprolactone n 20800gmol -1 , the dispersion PDI is 1.18.
PUM
| Property | Measurement | Unit |
|---|---|---|
| dispersity | aaaaa | aaaaa |
| dispersity | aaaaa | aaaaa |
| dispersity | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com