Preparation method of large-scale methanol catalyst
A catalyst and methanol conversion technology, which is applied in the preparation of organic compounds, physical/chemical process catalysts, chemical instruments and methods, etc., can solve the problems of low selectivity of dimethyl ether and ethanol, unsuitable for large-scale synthetic methanol production and use, etc. , to achieve the effect of improving methanol selectivity, good thermal conductivity, and improving high temperature performance
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
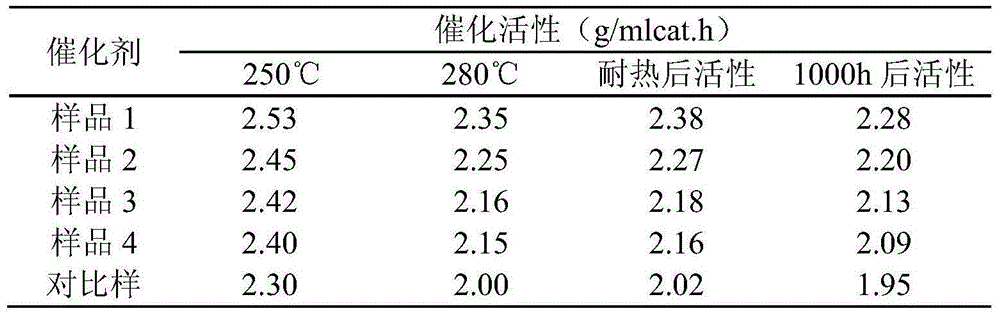
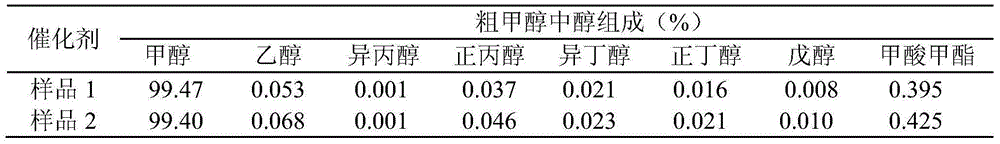
Embodiment 1
[0029] Weigh Mg(NO 3 ) 2 ·6H 2 O9.05g, Al(NO 3 ) 2 9H 2 O26.49g, dissolved in a 50ml desalinated beaker, stirred to make it completely dissolved, supplemented with desalted water and set the volume to 260ml, to prepare salt solution A. Weigh Na 2 CO 3 16.30g, dissolved in a 50ml desalinated beaker, stirred to dissolve completely, supplemented with desalinated water and set the volume to 260ml, to prepare alkaline solution A. Weigh Cu(NO 3 ) 2 ·3H 2 O147.92g, Zn(NO 3 ) 2 ·6H 2 O89.10g, Al(NO 3 ) 2 9H 2 O38.28g was dissolved in a 200ml desalinated beaker, stirred to make it completely dissolved, supplemented with desalted water and set the volume to 1000ml, and the salt solution B was prepared. Weigh Na 2 CO 3 123.66g, dissolved in a 200ml desalinated beaker, stirred to dissolve completely, supplemented with desalinated water and set the volume to 1000ml, to prepare alkaline solution B.
[0030]Preheat the salt solution A and alkali solution A to a reaction te...
Embodiment 2
[0034] Weigh Mg(NO 3 ) 2 ·6H 2 O13.08g, Al(NO 3 ) 2 9H 2 O38.28g, dissolved in a 50ml desalinated beaker, stirred to make it completely dissolved, supplemented with desalted water and constant volume to 375ml, prepared to obtain salt solution A. Weigh Na 2 CO 3 23.55g, dissolved in a 50ml desalinated beaker, stirred to dissolve completely, supplemented with desalinated water and set the volume to 375ml, to prepare alkaline solution A. Weigh Cu(NO 3 ) 2 ·3H 2 O147.92g, Zn(NO 3 ) 2 ·6H 2 O89.10g, Al(NO 3 ) 2 9H 2 O38.28g was dissolved in a 200ml desalinated beaker, stirred to make it completely dissolved, supplemented with desalted water and set the volume to 1000ml, and the salt solution B was prepared. Weigh Na 2 CO 3 123.66g, dissolved in a 200ml desalinated beaker, stirred to dissolve completely, supplemented with desalinated water and set the volume to 1000ml, to prepare alkaline solution B.
[0035] Preheat the salt solution A and alkali solution A to a ...
Embodiment 3
[0039] Weigh Sr(NO 3 ) 2 5.18g, Al(NO 3 ) 2 9H 2 O18.37g, dissolved in a 50ml desalinated beaker, stirred to make it completely dissolved, supplemented with desalted water and constant volume to 240ml, prepared to obtain salt solution A. Weigh Na 2 CO 3 11.30g, dissolved in a 50ml desalinated beaker, stirred to dissolve completely, supplemented with desalinated water and set the volume to 240ml, to prepare alkaline solution A. Weigh Cu(NO 3 ) 2 ·3H 2 O147.92g, Zn(NO 3 ) 2 ·6H 2 O89.10g, Al(NO 3 ) 2 9H 2 O38.28g was dissolved in a 200ml desalinated beaker, stirred to make it completely dissolved, supplemented with desalted water and set the volume to 1000ml, and the salt solution B was prepared. Weigh Na 2 CO 3 123.66g, dissolved in a 200ml desalinated beaker, stirred to dissolve completely, supplemented with desalinated water and set the volume to 1000ml, to prepare alkaline solution B.
[0040] Preheat the salt solution A and alkali solution A to a reaction ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com