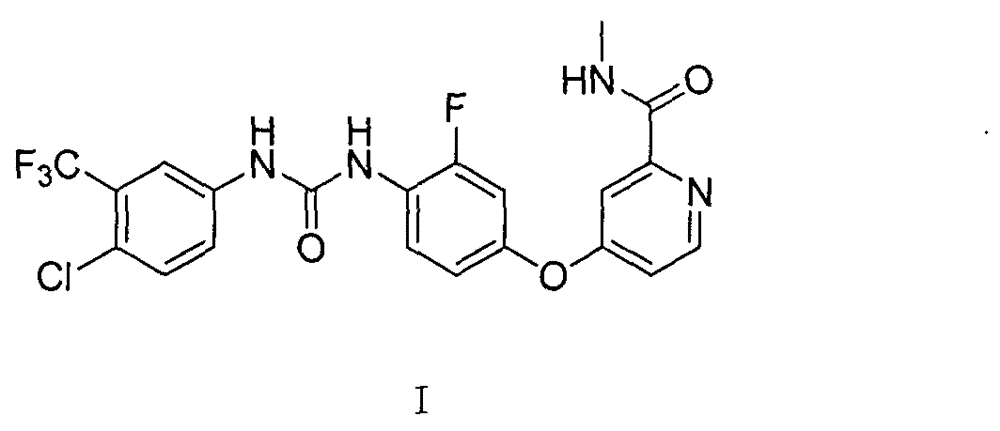
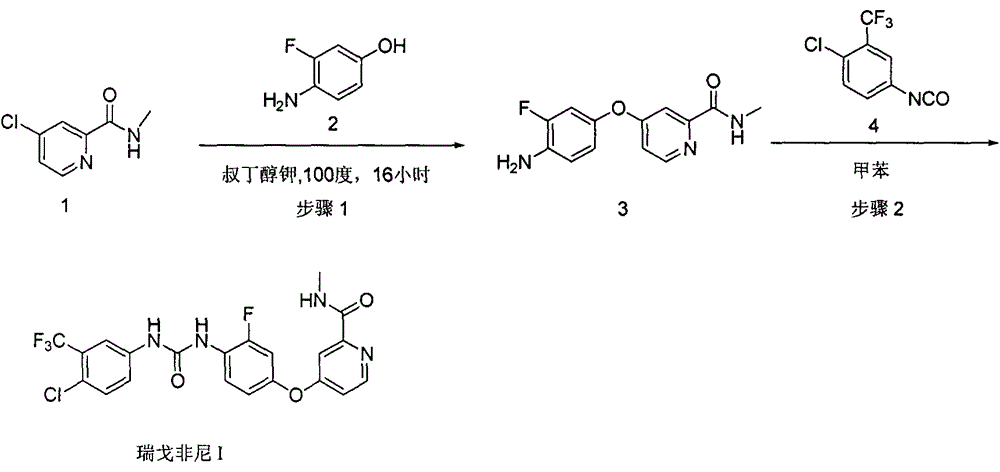
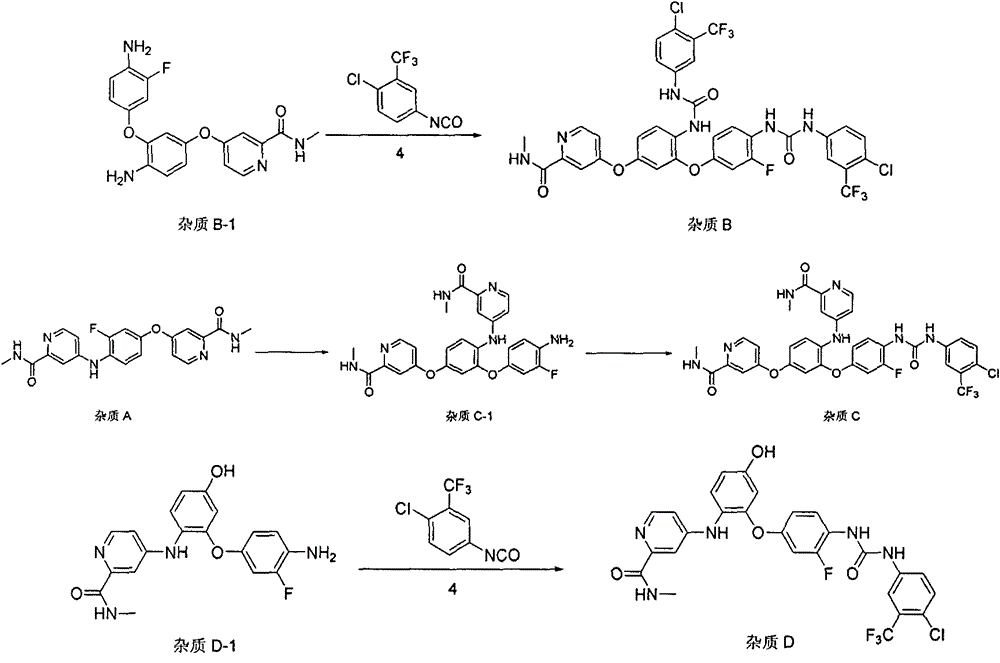
Preparation method for high-purity regorafenib
A high-purity, compound technology, applied in the field of regorafenib, can solve the problems of unfavorable industrialization, long crystallization cycle, cumbersome operation, etc., and achieve the effects of environmental friendliness, reduction of waste liquid discharge, and cost reduction
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0082] Example 1: Synthesis of 4-(4-chloro-3-fluorophenoxy)pyridine-2-carboxylic acid carboxamide (compound 3)
Embodiment 11
[0084]
[0085] Add N-methyl-4-chloropyridine-2-carboxamide (compound 1, 17g) and tetrabutylammonium bromide (3.22g) into 340ml tetrahydrofuran, and stir well. Sodium tert-butoxide (base 1, 10 g) was added. The temperature was raised to 50° C., 3-fluoro-4 aminophenol (compound 2, 13.9 g) was added, and the reaction was continued for 8 hours. After the reaction was completed, water was added to the reaction system to quench, and the reaction solution was extracted with dichloromethane. Separate the layers, collect the organic layer, and concentrate to obtain a residue. After dissolving the residue with dichloromethane, add methyl tert-butyl ether, stir, and filter after the solid precipitates, collect the filter cake, and dry to obtain 4-(4-chloro-3-fluorophenoxy)pyridine-2- Carboxylic acid formamide (compound 3).
[0086] Yield: 23g, 88.5%
[0087] Chemical purity (HPLC): 98.4%
[0088] MS: [M+1] + =262.09
Embodiment 12
[0090] Add N-methyl-4-chloropyridine-2-carboxamide (compound 1, 17g) and tetrabutylammonium bromide (16.1g) into 340ml of ethyl acetate, and stir well. Sodium hydroxide (base 1, 4 g) was added. The temperature was raised to 70° C., 3-fluoro-4 aminophenol (compound 2, 13.9 g) was added, and the reaction was continued for 8 hours. After the reaction was completed, water was added to the reaction system to quench, and the reaction solution was extracted with dichloromethane. Separate the layers, collect the organic layer, and concentrate to obtain a residue. After dissolving the residue with dichloromethane, add methyl tert-butyl ether, stir, and filter after the solid precipitates, collect the filter cake, and dry to obtain 4-(4-chloro-3-fluorophenoxy)pyridine-2- Carboxylic acid formamide (compound 3).
[0091] Yield: 24g, 91.9%
[0092] Chemical purity (HPLC): 98.3%
PUM
| Property | Measurement | Unit |
|---|---|---|
| purity | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com