Pharmaceutical erdosteine composite granules for treating respiratory tract infection
A technology of erdosteine and composition, which is applied in the field of erdosteine composition granules for the treatment of respiratory tract infection, and can solve the problems of drug absorption speed or degree, poor water solubility of erdosteine, and slow drug dissolution rate and other issues, to achieve the effect suitable for clinical application, good stability and high stability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0024] Example 1: Preparation of Erdosteine Crystals
[0025] Prepare a saturated methanol solution of erdosteine crude product at 30°C, then add a mixed solvent of ethanol and ether whose volume is 8 times the volume of the saturated methanol solution, the volume ratio of ethanol and ether is 3:1, and stir evenly, Stir while cooling down, the cooling rate is 15°C / hour, the stirring speed is 95 rpm, and at the same time, add propanol with a volume 4 times the volume of the mixed solvent of ethanol and ether, stop stirring after cooling to -5°C, and let it stand for cultivation. crystallized for 3 hours, filtered, and dried under reduced pressure to obtain erdosteine crystal compound.
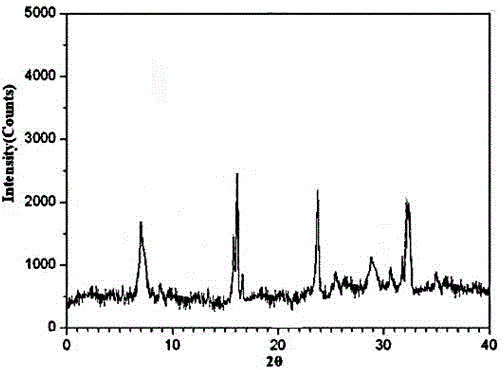
[0026] The X-ray powder diffraction pattern that the prepared erdosteine crystal uses Cu-Kα ray measurement to obtain is as follows figure 1 shown.
Embodiment 2
[0027] Example 2: Preparation of Erdosteine Granules
[0028] Prescription: 1.5 parts by weight of the erdosteine crystal compound prepared in Example 1, 1.2 parts by weight of sucrose, 0.3 parts by weight of lactitol, 0.02 parts by weight of vanillin, and 0.5 parts by weight of purified water.
[0029] Preparation:
[0030] (1) Processing of raw and auxiliary materials: sieve erdosteine, lactitol, and vanillin to 80 meshes;
[0031] (2) Weighing: Weighing according to the prescription;
[0032] (3) Granulation: add erdosteine, sucrose, lactitol, and vanillin to the wet mixing granulator, and dry mix for 10 minutes; add wetting agent, purified water, wet mix and cut for 1-1.5min, 16 Granulated soft materials;
[0033] (4) Drying and sieving: distribute the wet granules obtained from granulation evenly on the baking tray of the oven, set the temperature at 55-65°C, and the total drying time is 2.5-3.0 hours, and sieve the dried material to 40 meshes for granulation;
...
Embodiment 3
[0036] Example 3: Preparation of Erdosteine Granules
[0037] Prescription: 1.5 parts by weight of the erdosteine crystal compound prepared in Example 1, 1.3 parts by weight of sucrose, 0.4 parts by weight of lactitol, 0.03 parts by weight of vanillin, and 0.6 parts by weight of purified water.
[0038] Preparation:
[0039] (1) Processing of raw and auxiliary materials: sieve erdosteine, lactitol, and vanillin to 80 meshes;
[0040] (2) Weighing: Weighing according to the prescription;
[0041] (3) Granulation: add erdosteine, sucrose, lactitol, and vanillin to the wet mixing granulator, and dry mix for 10 minutes; add wetting agent, purified water, wet mix and cut for 1-1.5min, 16 Granulated soft materials;
[0042] (4) Drying and sieving: distribute the wet granules obtained from granulation evenly on the baking tray of the oven, set the temperature at 55-65°C, and the total drying time is 2.5-3.0 hours, and sieve the dried material to 40 meshes for granulation;
...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com