Arylmethyl phosphonate preparation method
A technology of arylmethyl phosphonate and arylmethyl alcohol, applied in the field of organic synthesis, can solve the problems of high cost, environmental pollution, high toxicity of reagents and the like, and achieves the effects of simple operation, high product quality and reduction of reaction cost.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
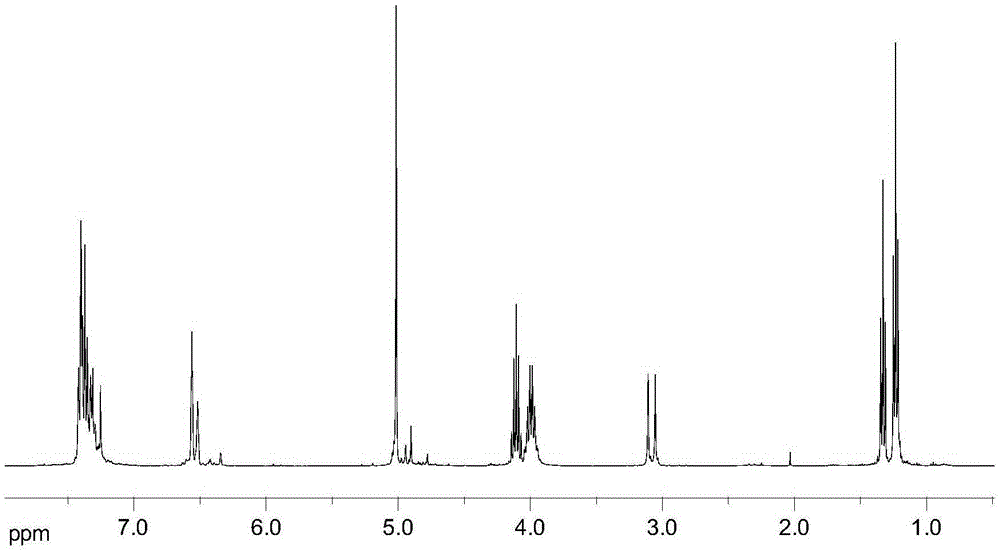
[0021] With 6.40g (0.02mol) 3,5-dibenzyloxybenzyl alcohol, 9.96g (0.06mol) triethyl phosphite, 1.09g (3.0mmol) cetyltrimethylammonium bromide and 0.07g ( 0.4mmol) KI was added to the three-necked flask, stirred thoroughly and reacted at 140°C for 6h, then depressurized (pressure 10mmHg, the same as the following examples) to distill off excess triethyl phosphite, and continued to react at 140°C for 4h. After cooling down to room temperature, extract with a mixed solvent of ethyl acetate and petroleum ether (boiling range: 60-90°C, the same as the following examples) with a volume ratio of 1:3, and concentrate under reduced pressure to obtain 7.95 g of the product, of which 3,5-di The yield of diethyl benzyloxybenzylphosphonate was 60.3%. 1 H-NMR (CDCl 3 ,400MHz)δ:1.23~1.26(t,J=7.0Hz,6H,Ar-P-O-CH 2 -CH 3 ), 1.32~1.35 (t, J=7.2Hz, 9H, P-O-CH 2 -CH 3 ), 3.09 (d, 2H, J=21.6Hz, Ar-CH 2 -P),3.96~4.05(m,4H,Ar-P-O-CH 2 ),4.08~4.15(m,6H,P-O-CH 2 ),5.04(s,4H,PhCH 2 ),6.52~6.57(...
Embodiment 2
[0024] With 12.80g (0.04mol) 3,5-dibenzyloxybenzyl alcohol, 33.20g (0.20mol) triethyl phosphite, 1.92g (6.0mmol) cetyltrimethylammonium chloride and 0.13g ( 0.8mmol) KI was added into the three-necked flask, stirred thoroughly and reacted at 185°C for 5.5h, distilled off excess triethyl phosphite, and continued to react at 185°C for 4h. After cooling down to room temperature, it was extracted with a mixed solvent of ethyl acetate and petroleum ether with a volume ratio of 1:3, and concentrated under reduced pressure to obtain 20.03 g of the product, of which the yield of diethyl 3,5-dibenzyloxybenzylphosphonate was was 75.8%.
Embodiment 3
[0026] With 6.40g (0.02mol) 3,5-dibenzyloxybenzyl alcohol, 26.56g (0.16mol) triethyl phosphite, 0.32g (1.0mmol) tetrabutylammonium bromide and 0.06g (0.4mmol) NaI Add it into a three-necked flask, stir it well, and react it at 162°C for 6h, steam off excess triethyl phosphite, and continue to react at 162°C for 4h. After cooling down to room temperature, it was extracted with a mixed solvent of ethyl acetate and petroleum ether with a volume ratio of 1:3, and concentrated under reduced pressure to obtain 11.25 g of the product, of which the yield of diethyl 3,5-dibenzyloxybenzylphosphonate was was 85.3%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com