Long-chain alkyl imidazole phosphate ionic liquid, preparation method and application thereof
A technology of long-chain alkylimidazole phosphate and diisooctyl imidazole phosphate, which is applied in the field of long-chain alkylimidazole phosphate ionic liquid and its preparation and application, and can solve catalyst residue, product performance impact, side reactions, etc. problems, to achieve the effect of improving tribological properties, reducing costs, and excellent anti-friction and anti-wear effects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
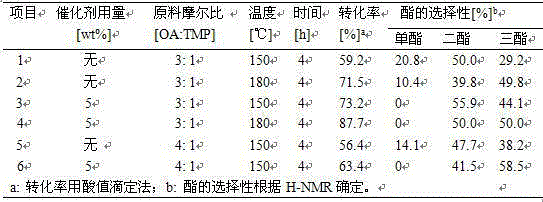
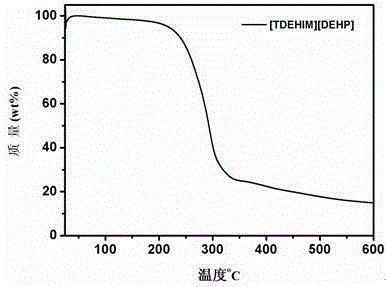
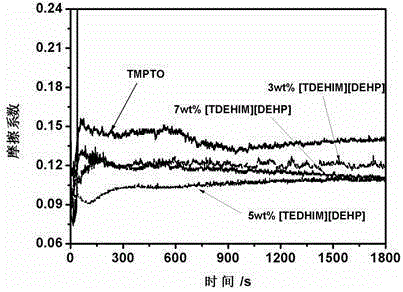
[0026] While stirring constantly, equimolar imidazole and sodium hydroxide were mixed at 110 o C reacts for 24h to generate imidazole sodium (NaIm); using tetrahydrofuran as solvent, imidazole sodium and equimolar tetradecane bromide at 65 o C was refluxed for 24 hours, the sodium bromide generated was filtered out, and the filtrate was rotary evaporated to obtain the crude product N-tetradecylimidazole. The crude product was dissolved in dichloromethane, and activated carbon and anhydrous magnesium sulfate were added successively to remove impurities. Filtration, most of the dichloromethane was removed by rotary evaporation, leaving a small amount at 60 o C was removed by vacuum drying to finally obtain the desired product N-tetradecylimidazole. Under a nitrogen atmosphere, the N-tetradecyl imidazole obtained above was added to equimolar triisooctyl phosphate, 150 o C reacted for 12 h under the protection of nitrogen to obtain a light yellow ionic liquid [TDEHIM][DEHP] wit...
Embodiment 2
[0028] While stirring constantly, equimolar imidazole and sodium hydroxide were mixed at 110 o C reacts for 24h to generate imidazole sodium (NaIm); using tetrahydrofuran as solvent, imidazole sodium and equimolar tetradecane bromide at 65 o C was refluxed for 14 hours, the generated sodium bromide was removed by filtration, and the filtrate was rotary evaporated to obtain the crude product N-tetradecylimidazole. The crude product was dissolved in dichloromethane, and activated carbon and anhydrous magnesium sulfate were added successively to remove impurities. Filtration, most of the dichloromethane was removed by rotary evaporation, leaving a small amount at 60 o C was removed by vacuum drying to finally obtain the desired product N-tetradecylimidazole. Under a nitrogen atmosphere, the N-tetradecyl imidazole obtained above was added to equimolar triisooctyl phosphate, 150 o C reacted for 12 h under the protection of nitrogen to obtain light yellow ionic liquid N-tetradecy...
Embodiment 3
[0030] While stirring constantly, equimolar imidazole and sodium hydroxide were mixed at 110 o C reacts for 24h to generate imidazole sodium (NaIm); using tetrahydrofuran as solvent, imidazole sodium and equimolar tetradecane bromide at 65 o C was refluxed for 18 hours, and the sodium bromide generated was filtered out, and the filtrate was rotary evaporated to obtain the crude product N-tetradecylimidazole. The crude product was dissolved in dichloromethane, and activated carbon and anhydrous magnesium sulfate were added successively to remove impurities. Filtration, most of the dichloromethane was removed by rotary evaporation, leaving a small amount at 60o C was removed by vacuum drying to finally obtain the desired product N-tetradecylimidazole. Under a nitrogen atmosphere, the N-tetradecyl imidazole obtained above was added to equimolar diisooctyl phosphate, 150 o C reacted for 12 h under the protection of nitrogen to obtain a light yellow ionic liquid [TDEHIM][DEHP] wi...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com