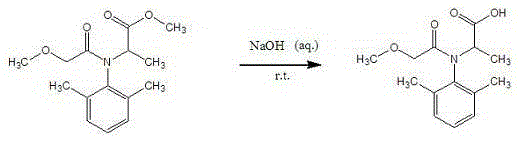Test paper for detecting metalaxyl residue and application thereof
A metalaxyl and test paper technology, which is applied in the field of test paper for the detection of metalaxyl residues, can solve the problems of tedious and time-consuming test sample processing, and achieve the effects of short detection time, wide application range and simple operation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0040] The preparation of embodiment 1 metalaxyl detection test paper
[0041] The preparation method of the test paper mainly includes the following steps:
[0042] 1) Prepare a conjugate release pad coated with a metalaxyl monoclonal antibody-colloidal gold marker;
[0043] 2) Prepare a reaction membrane with a detection line coated with a metalaxyl hapten-carrier protein conjugate and a quality control line coated with a goat anti-mouse antibody;
[0044] 3) Assemble the conjugate release pad, reaction membrane, sample absorption pad, water absorption pad and PVC bottom plate prepared in 1) and 2) into a test paper.
[0045] The following is a step-by-step detailed description:
[0046] 1. Preparation of metalaxyl hapten
[0047] Metalaxyl was dissolved in ethanol, 10% NaOH aqueous solution was added with stirring at room temperature, and the reaction was monitored by TLC until there was no raw material or the raw material point was very shallow. The reaction was stopped...
Embodiment 2
[0081] The detection of metalaxyl residue in the sample of embodiment 2
[0082] 1. Sample pretreatment
[0083] Take 1.0g±0.05g of homogeneous sample into a 50ml polystyrene centrifuge tube, add 5ml of extract, shake with a shaker for 5min, centrifuge at 3000g for 5min at room temperature, and take the supernatant for testing.
[0084] 2. Test with test paper
[0085] Use a pipette to suck up the sample solution to be tested and add 2-3 drops vertically to the sample addition hole. When the liquid flows, start timing and react for 5 minutes. The result is judged, and other times are judged invalid.
[0086] 3. Analysis of test results
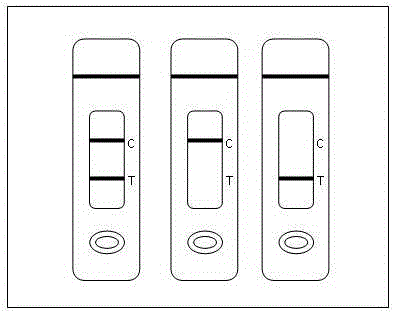
[0087] Negative (-): Both the T line and the C line are colored, indicating that the metalaxyl concentration in the sample is below the detection limit, such as image 3 .
[0088] Positive (+): T line has no color and C line has color, indicating that the drug concentration of metalaxyl in the sample is equal to or higher than the detectio...
Embodiment 3
[0090] Example 3 Sample Detection
[0091] 1. Detection limit test
[0092] A blank tobacco sample was taken, and metalaxyl was added to it to a final concentration of 1, 2, and 4 mg / kg, respectively. Test paper was taken for detection, and the measurement was repeated three times for each sample.
[0093] When testing tobacco, tea, and vegetable samples with test paper, when the metalaxyl concentration is 1mg / kg, the test paper shows two red lines visible to the naked eye, which is negative; when the metalaxyl concentration is 2, 4mg / kg, the quality control line of the test paper is displayed, but the detection line is not displayed, which is positive; it indicates that the detection line of this test paper to metalaxyl is 2 mg / kg.
[0094] 2. False positive rate and false negative rate test
[0095] Take 20 positive tobacco samples with known metalaxyl content greater than 2 mg / kg and 20 negative tobacco samples with known metalaxyl content less than 2 mg / kg, and use thre...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com