Preparation method of super-high-quantum-yield carbon quantum dots with citric acid-urea as raw materials
A quantum yield, carbon quantum dot technology, applied in chemical instruments and methods, luminescent materials, etc., can solve the problems of uncontrollable microwave temperature, low fluorescence efficiency, low fluorescence yield, etc. Inexpensive and readily available materials, and the effect of high fluorescence stability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
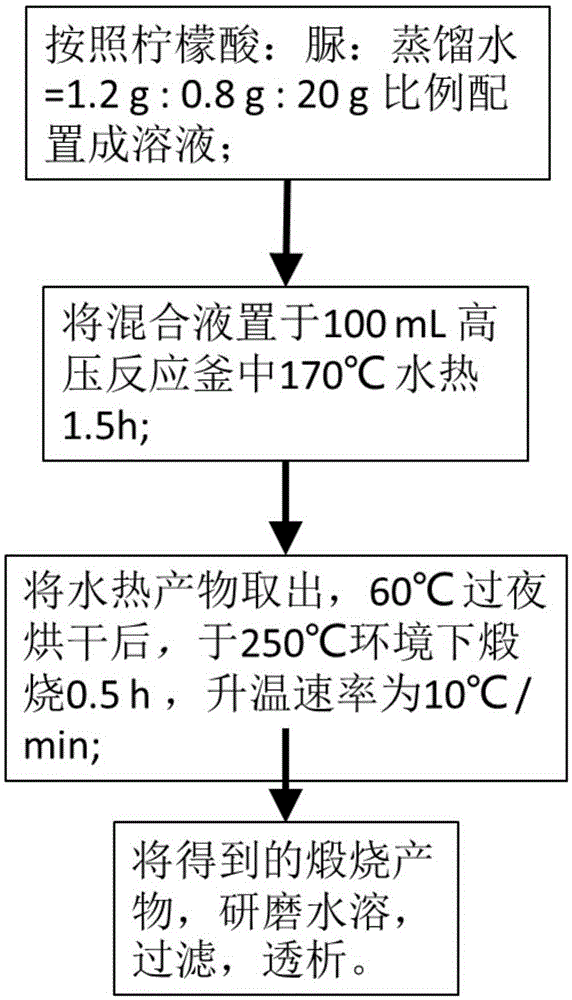
[0029] (1) Mix citric acid, urea and distilled water (the mass ratio is 1.2g:0.8g:20g), stir and dissolve to obtain a mixed solution;
[0030] (2) Place the mixture in (1) in a 100mL autoclave at 170°C for a hydrothermal reaction for 1.5 hours to obtain a hydrothermal product;
[0031] (3) Dry the hydrothermal product obtained in (2) overnight at 60°C;
[0032] (4) Place the oven-dried product obtained in (3) in an environment of 250°C and calcinate for 0.5h at a heating rate of 10°C / min to obtain the calcined product;
[0033] (5) Grind the calcined product obtained in (4) to fully dissolve in distilled water, and filter with a 0.22 μm water-based filter membrane to obtain a carbon dot solution with uniform particle size.
[0034] The preparation method flow chart is as figure 1 Shown. figure 2 It is a transmission electron microscope image of the fluorescent quantum dots of carbon particles in Example 1, and the average particle size of the particles is about 6.64 nm. image 3 It is...
Embodiment 2
[0036] (1) Mix citric acid, urea and distilled water (the mass ratio is 1.2g:0.8g:25g), stir and dissolve to obtain a mixed solution;
[0037] (2) Place the mixture in (1) in a 100mL autoclave at 170°C for a hydrothermal reaction for 1.5 hours to obtain a hydrothermal product;
[0038] (3) Dry the hydrothermal product obtained in (2) overnight at 60°C;
[0039] (4) Place the oven-dried product obtained in (3) in an environment of 250°C and calcinate for 0.5h at a heating rate of 10°C / min to obtain the calcined product;
[0040] (5) Grind the calcined product obtained in (4) to fully dissolve in distilled water, and filter with a 0.22 μm water-based filter membrane to obtain a carbon dot solution with uniform particle size.
[0041] Image 6 It is a comparison diagram of the fluorescence intensity of carbon particle fluorescent quantum dots under different environmental pH. It can be seen from the figure that the fluorescence intensity of the carbon dot solution is the strongest when th...
Embodiment 3
[0043] (1) Mix citric acid, urea and distilled water (the mass ratio is 1.2g:0.8g:20g), stir and dissolve to obtain a mixed solution;
[0044] (2) Place the mixture in (1) in a 100mL autoclave at 170°C for a hydrothermal reaction for 0.5 to 3 hours to obtain a hydrothermal product;
[0045] (3) Dry the hydrothermal product obtained in (2) overnight at 60°C;
[0046] (4) Place the oven-dried product obtained in (3) to calcinate for 1 hour at a temperature of 300°C with a heating rate of 10°C / min to obtain the calcined product;
[0047] (5) Grind the calcined product obtained in (4) to fully dissolve in distilled water, and filter with a 0.22 μm water-based filter membrane to obtain a carbon dot solution with uniform particle size.
[0048] Figure 5 Fluorescence intensity of citric acid-urea (urea) carbon particles fluorescent quantum dots prepared by a two-step hydrothermal-pyrolysis method under different hydrothermal time (0.5h, 1.0h, 1.5h, 2.0h, 2.5h, 3.0h) Comparing the graph, it c...
PUM
| Property | Measurement | Unit |
|---|---|---|
| The average particle size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com