A kind of synthetic penicillin g acylase mutant and its application in the preparation of amoxicillin
An amoxicillin and penicillin technology, which is applied in the fields of genetic engineering and enzyme engineering, can solve the problems of weak acid resistance and low synthesis activity of mutant enzymes, and achieve the effects of low hydrolysis activity, good synthesis activity and high hydrolysis ratio.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0076] Embodiment 1: Preparation of SPGA mutant with low hydrolytic activity and high synthetic activity
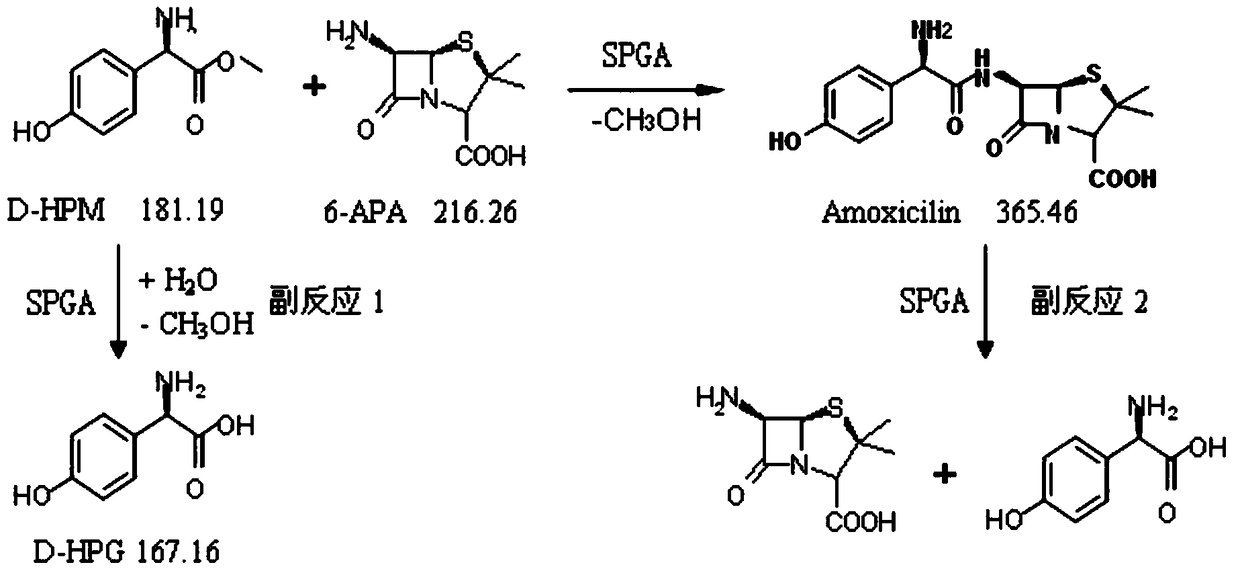
[0077] Depend on figure 1The reaction formula shows that SPGA can not only catalyze the synthetic product amoxicillin from the parent nucleus 6-aminopenicillanic acid (6-APA) and the side chain D-hydroxyphenylglycine methyl ester (D-HPM), but also hydrolyze the product to generate parent Core and side chain, the reversibility of the reaction is determined by the equilibrium constant of the reaction; at the same time, p-hydroxyphenylglycine methyl ester can be hydrolyzed into p-hydroxyphenylglycine by SPGA, therefore, the hydrolysis activity of SPGA is reduced and its synthesis activity is improved is of great significance.
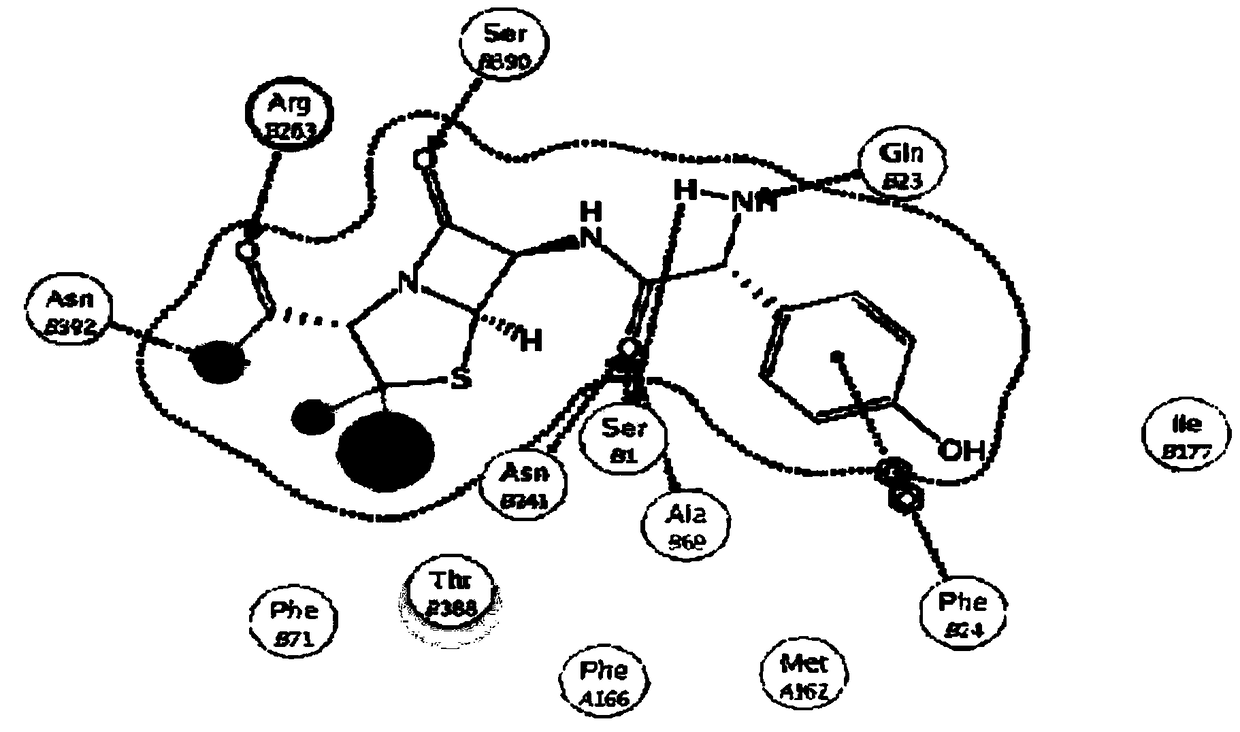
[0078] 1-1 Determine the mutation site by computer simulation
[0079] 1-1-1 SPGA 3D structure modeling
[0080] Using penicillin G acylase derived from Escherichia coli (E.coli) and Alcaligenes faecalis (A.faecalis) whose three-dimensional struct...
Embodiment 2
[0109] Embodiment 2: the preparation of acid-resistant SPGA mutant
[0110] Because the enzymatic synthesis of amoxicillin is carried out under acidic pH conditions, and because the penicillin G acylase needs to be used continuously for 5 to 10 days in the industrial production process, this requires the enzyme to be stable and not easy to produce under acidic pH conditions. Inactivation, and the acid resistance of wild-type penicillin G acylase is poor, so it is urgent to develop a new type of PGA resistant to acidic pH. In order to solve this difficult problem in industrial production, the inventors set up a new development route to further modify the above-mentioned SPGA-2 mutant with the best effect.
[0111] 2-1 Construction of acid-resistant SPGA random mutant library
[0112] In order to improve the acid resistance of SPGA, the inventor used the SPGA-2 template, wherein the primers were T7 universal primers (SEQ IDNO: 13 and 14), to construct a random mutant library by...
Embodiment 3
[0123] Example 3 Separation, purification and immobilization of recombinant SPGA protein
[0124] 3-1 Separation and purification of recombinant SPGA protein
[0125] Since the two His-tags at the N-terminal and C-terminal in the prokaryotic expression vector pET28b(+) were introduced during the construction of the expression vector, the inventors used the histidine tag to carry out Immobilized Metal Chelate Affinity Chromatography (IMAC) To purify the recombinant protein, the specific method is as follows.
[0126] Take 100mL of the SPGA fermentation broth after overnight induction, centrifuge and discard the supernatant to collect the bacteria (10000rpm, 4°C, 10min), wash the bacteria twice with phosphate buffer (pH 8.0, 0.1mol / L), and collect by centrifugation The cells were concentrated 5 times and resuspended in 20ml of phosphate buffer (pH 8.0, 0.1mol / L). Put the above-mentioned treated bacterial solution in ice water and perform ultrasonic crushing until clarificatio...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com