Polymer lipid sphere carrying active drugs and preparation method thereof
A technology for active drugs and polymers, applied in the field of medicine, can solve the problems of difficulty in researching the relationship between microsphere particle size and drug efficacy, difficulty in screening particle size requirements, and inability to prepare particle size, etc., to ensure repeatability and size. Uniform and maintain the effect of drug activity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
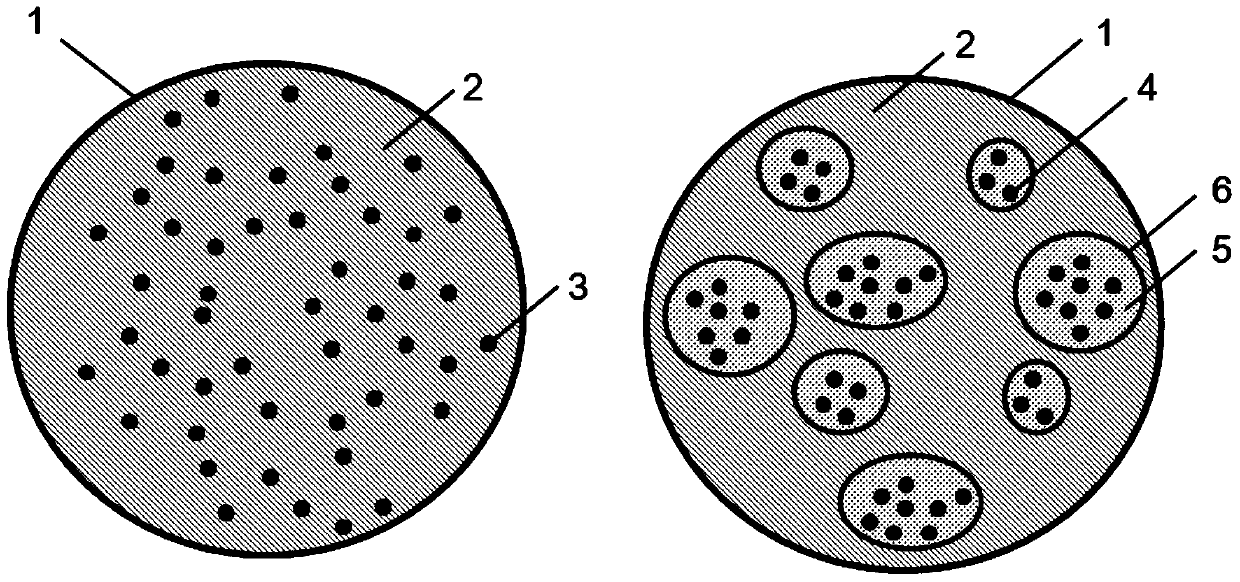
[0070] A microporous membrane with a pore size of 9.2 μm is used to prepare PLGA microspheres loaded with hydrophilic proteins. The specific implementation method is as follows: figure 1 Shown: Accurately weigh 10 mg bovine serum albumin and dissolve it in 500 μL deionized water as the inner water phase (W 1 ); 160mgPLGA is added to 5mL dichloromethane solution and it is fully dissolved, as the oil phase (O); the aqueous solution that gets volume is 50mL containing 1%PVA as the external water phase (W 2 ). Add the inner water phase to the oil phase and homogeneously emulsify (20000rpm, 1min) to form water-in-oil (W 1 / O) type colostrum, then add the colostrum into the external water phase, mechanically stir (300rpm, 1min) to form W 1 / O / W 2 Type double milk. The double emulsion was poured into a rapid membrane emulsification reaction tank, and passed through the membrane 5 times under the action of nitrogen pressure of 0.1MPa to form an emulsion with uniform particle size....
Embodiment 2
[0072] A microporous membrane with a pore size of 9.2 μm is used to prepare polymer lipid spheres loaded with hydrophilic proteins. The specific implementation method is as follows figure 1 Shown: Accurately weigh 10 mg transferrin and dissolve in 500 μL deionized water as the inner water phase (W 1 ); 20mgHSPC (hydrogenated soybean lecithin) and 140mgPLGA were added to 5mL methylene chloride solution to make it fully dissolved, as the oil phase (O); the volume was 50mL containing 1%PVA aqueous solution as the external water phase (W 2 ). Add the inner water phase to the oil phase and homogeneously emulsify (20000rpm, 1min) to form water-in-oil (W 1 / O) type colostrum, then add the colostrum into the external water phase, mechanically stir (300rpm, 1min) to form W 1 / O / W 2 Type double milk. The double emulsion was poured into a rapid membrane emulsification reaction tank, and passed through the membrane 5 times under the action of nitrogen pressure of 0.1MPa to form an emu...
Embodiment 3
[0074] A microporous membrane with a pore size of 9.2 μm is used to prepare polymer lipid spheres loaded with hydrophilic proteins. The specific implementation method is as follows figure 1 Shown: Accurately weigh 10 mg of ovalbumin as the solid phase (S); add 40 mg of HSPC and 120 mg of PLGA to 5 mL of dichloromethane solution to fully dissolve it as the oil phase (O); take a volume of 100 mL containing 0.8% PVA aqueous solution as the external aqueous phase (W 2 ). Disperse the protein solid phase S in the oil phase, and homogeneously emulsify (20000rpm, 1min) to form a solid-in-oil (S / O) emulsion system, then add it to the external water phase, and mechanically stir (300rpm, 1min) , forming S / O / W2 double emulsion. The double emulsion was poured into a rapid membrane emulsification reaction tank, and passed through the membrane 4 times under the action of nitrogen pressure of 0.05MPa to form an emulsion with uniform particle size. It was mechanically stirred and solidified...
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle size | aaaaa | aaaaa |
| pore size | aaaaa | aaaaa |
| pore size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com