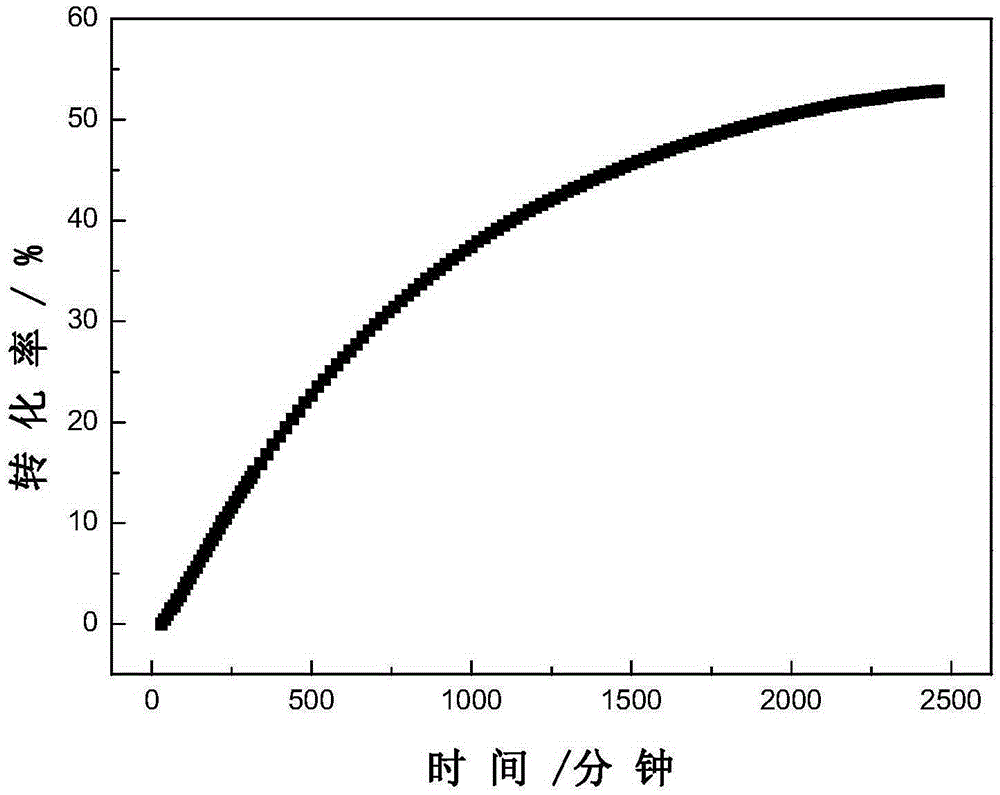
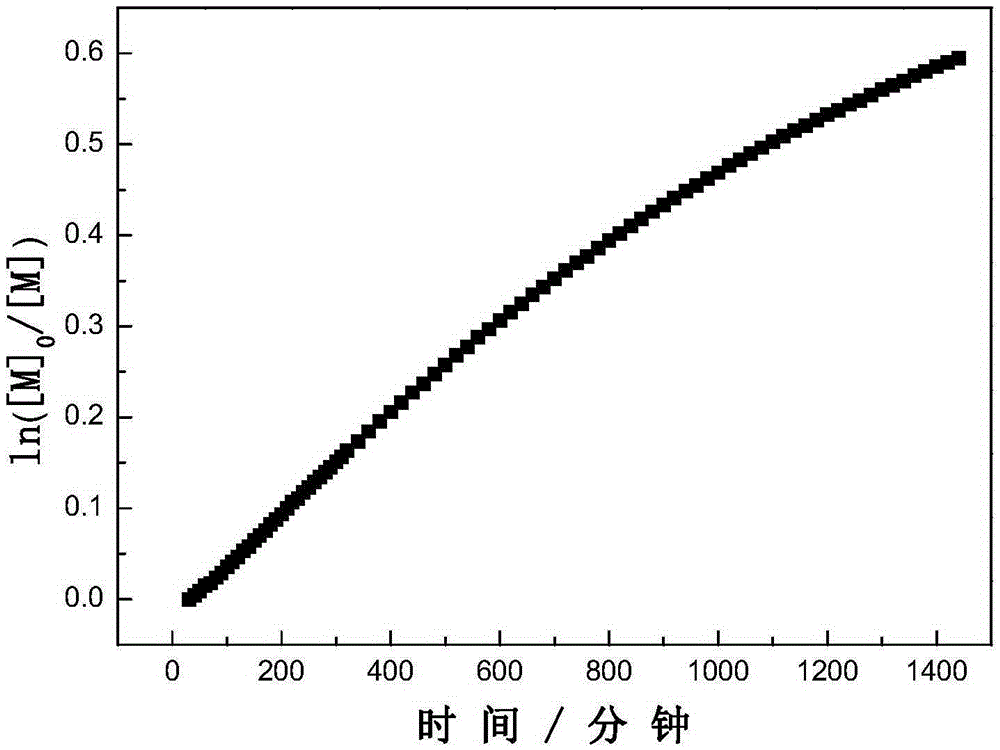
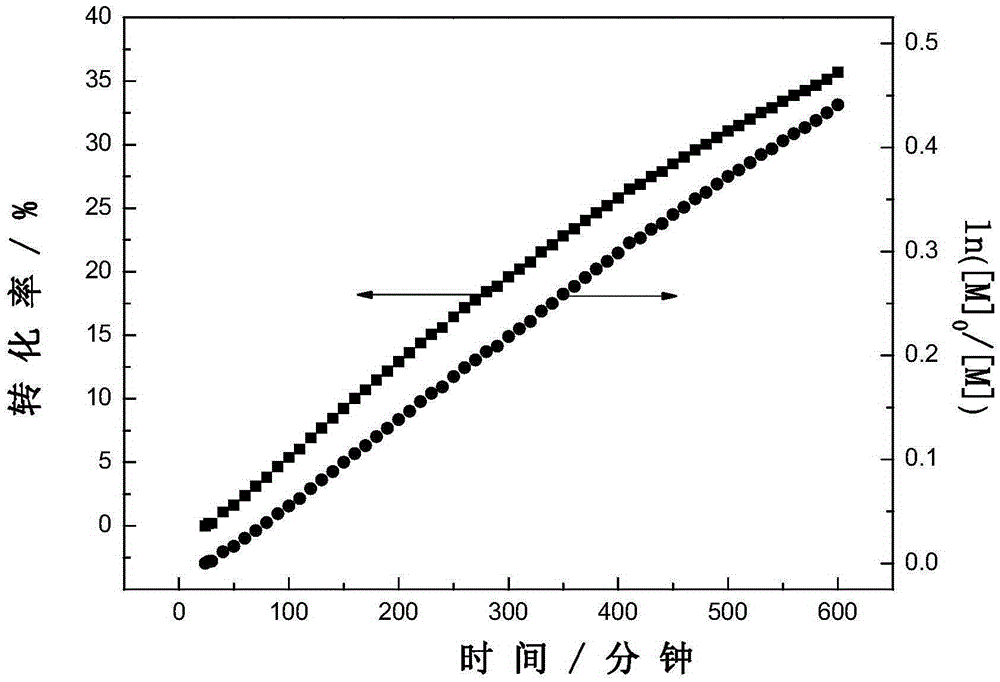
Dithiocarboxylic acid pentafluorobenzyl ester, preparation method and application thereof
A technology of pentafluorobenzyl ester and dithiocarboxylic acid, applied in the direction of organic chemistry, bulk chemical production, etc., can solve the problems of difficult to effectively control high fluorine-containing monomers, unstable end groups of polymer products, etc., and achieve structural Excellent controllability, good compatibility or affinity, and good solubility
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0027] Synthesis of pentafluorobenzyl dithiopentafluorobenzoate with the following structural formula
[0028]
[0029] Dissolve 1.75mL (14mmol) of pentafluorobromobenzene in 30mL of tetrahydrofuran, and then, under anhydrous and oxygen-free conditions, add 0.36g (15mmol) of magnesium powder and iodine grains (2 to 3 grains) to a three-necked flask gradually. Add the tetrahydrofuran solution of pentafluorobromobenzene dropwise. After the purple (color of iodine) in the reaction mixture disappears, raise the temperature to 70° C. and add the remaining pentafluorobromobenzene dropwise to the three-necked flask under stirring. Tetrahydrofuran solution, control the addition rate to keep the system in a slightly boiling state, continue to react at 70°C for 2 hours after the dropwise addition, so that the reaction of the magnesium powder is completely or basically disappeared, and the Grignard reagent is prepared. Cool the prepared Grignard reagent to 40°C, add 1.14g (15mmol) car...
Embodiment 2
[0031] In embodiment 1, the consumption of carbon disulfide is increased to 1.52g (20mmol), and other steps are identical with embodiment 1, obtain dithiopentafluorobenzoic acid pentafluorobenzyl ester, and its yield is 76% (data after column chromatography separation) ). The appearance, melting point and spectral data of the product are the same as in Example 1.
Embodiment 3
[0033] In Example 1, 1.75mL (14mmol) of pentafluorobromobenzene was dissolved in 15mL of tetrahydrofuran, and then, under anhydrous and oxygen-free conditions at 40°C, 3.73g (28mmol) of ethylmagnesium bromide and 30mL Add tetrahydrofuran solution of pentafluorobromobenzene dropwise to a three-necked tetrahydrofuran flask. After the dropwise addition, raise the temperature to 50°C for 2 hours of reaction. During the reaction process, continuously distill off the produced bromoethane to prepare the Grignard reagent. The other steps were the same as in Example 1 to obtain pentafluorobenzyl dithiopentafluorobenzoate with a yield of 71% (data after separation by column chromatography). The appearance, melting point and spectral data of the product are the same as in Example 1.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com