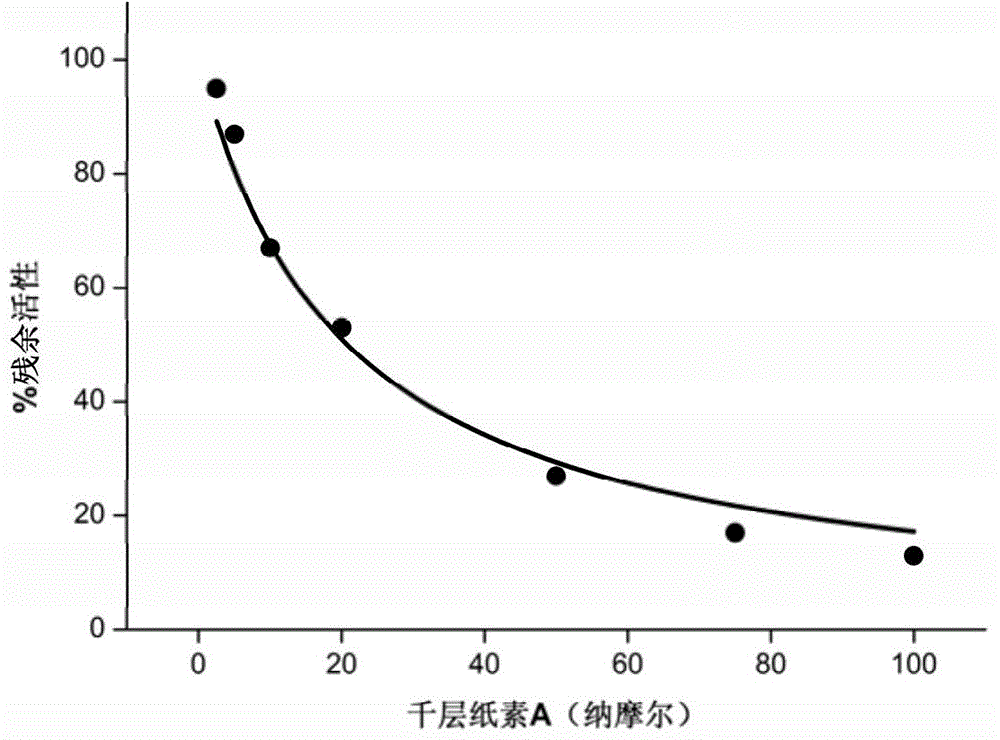
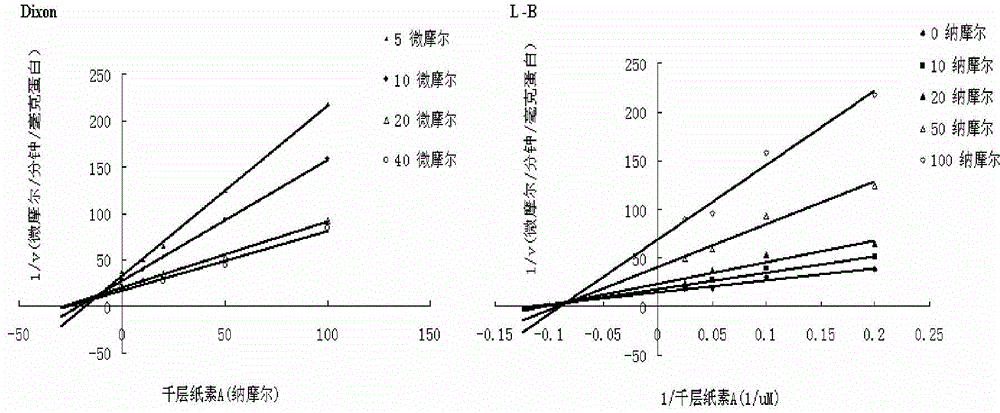
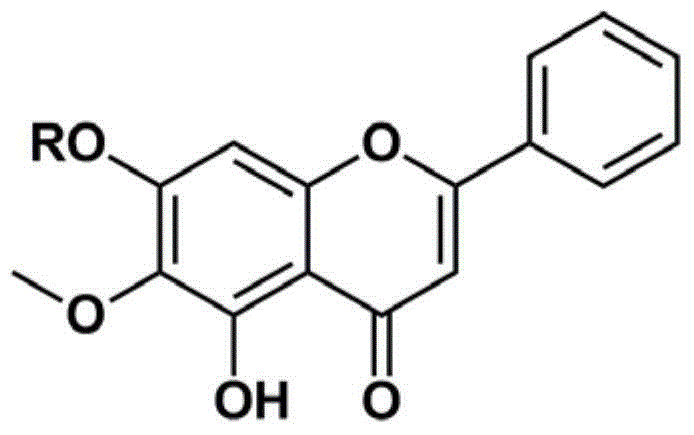
Application of oroxylin A and pro-drug thereof as catechol-type medicine synergist
A technology of phyllophyllin and prodrugs, which is applied in the field of medicine, can solve the problems of low inhibitory activity, poor membrane penetration ability, poor oral bioavailability, etc., and achieve good safety and high safety effects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0026] Preparation of kelplin A-7-acetyl ester
[0027]
[0028] Weigh 2.0g of phyllophyllin A and place it in a 100ml three-neck flask, add 20ml of dichloromethane to dissolve, add 2ml of pyridine and 1.5ml of acetic anhydride under stirring, react at 25°C for 8h, and detect the end of the reaction by TLC. Add 30ml of water to quench the reaction, extract with 50mlx3 dichloromethane, combine the dichloromethane phases, wash 30mlx2 with water, dry over anhydrous sodium sulfate, filter, evaporate the solvent to dryness under reduced pressure, and separate the residue by silica gel column chromatography to obtain the compound Melaleuca paper Preparation of Vetin A-7-Acetyl Ester 1.6g, pale yellow solid.
[0029] 1 HNMR (400MHz, d-DMSO) δ: 2.31 (s, 3H), 3.93 (s, 3H), 7.09 (d, J = 4Hz, 2H), 7.58-7.64 (m, 3H), 8.13 (d, J = 8Hz, 2H), 12.87(s, 1H);
[0030] 13 CNMR (100MHz, d-DMSO) δ: 20.48, 57.36, 92.26, 105.56, 105.67, 123.01, 127.01, 129.65, 131.01, 132.76, 151.94, 154.92, ...
Embodiment 2
[0032] Preparation of kelplin A-7-butyryl ester
[0033]
[0034] Weigh 1.0g of phyllophyllin A and place it in a 50ml three-necked bottle, add 10ml of dichloromethane to dissolve, add 1.5ml of triethylamine under stirring, slowly add 0.6ml of butyryl chloride dropwise in an ice-water bath, and react at 5-10°C After 6 h, TLC detected that the reaction was complete. Add 20ml of ice water to quench the reaction, extract with 30mlx3 dichloromethane, combine the dichloromethane phases, wash 20mlx2 with water, dry over anhydrous sodium sulfate, filter, evaporate the solvent to dryness under reduced pressure, and separate the residue by silica gel column chromatography to obtain compound Melaleuca Preparation of paperin A-7-butyryl ester 780mg, pale yellow solid.
[0035] 1 HNMR (400MHz, d-DMSO) δ: 0.96(t, J=7.2Hz, 3H), 1.64(q, J=7.6Hz, 2H), 2.37(t, J=7.2Hz, 2H), 3.91(s, 3H), 7.10(d, J=4Hz, 2H), 7.59-7.66(m, 3H), 8.12(d, J=8Hz, 2H), 12.91(s, 1H);
[0036] 13 CNMR (100MHz, d-...
Embodiment 3
[0038] Preparation of kelplin A-7-glucuronic acid
[0039]
[0040] Melaleucain A-7-glucuronic acid is a metabolite of glucuronidation metabolism of Melaleucain A. For this, the use of human liver microsomes as the enzyme source combined with biological preparation and fast chromatography technology A-7-glucuronic acid is prepared, and the specific experimental process is as follows:
[0041] (1) Add the following solutions to a 1.5ml EP tube: 815μl of Tris-HCl (50mM), 100μl of MgCl2 (150mM), 25μl of HLM (20mg / ml), 10μl of Melaleucain A (100mM), shake and mix;
[0042] (2) Pre-incubate the EP tube at 37°C for 3 minutes, add 50 μl of UDPGA (40mM), shake and mix, and react at 37°C;
[0043] (3) After reacting for 4 hours, add 500 μl of acetonitrile to the reaction system to terminate the reaction;
[0044] (4) Centrifuge at 20000g / min for 20min at 4°C, and collect the supernatant in a 50ml centrifuge tube;
[0045] (5) Load the sample on the SPE column, wash with water, wash...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com