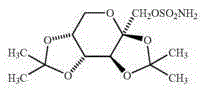
Topiramate sustained release preparation pharmaceutical composition
A technology of sustained-release preparations and topiramate, which is applied in the pharmaceutical composition of topiramate sustained-release preparations and its preparation field, can solve the problems of complex preparation process of cyclodextrin inclusion compound, increase the risk of drug degradation, reduce drug degradation, etc., and achieve stable and stable production. Effect of effective blood concentration, reduction of incidence, and reduction of side effects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1-4
[0026] Embodiment 1-4 sustained-release granule prescription:
[0027] Material name effect Example 1(g) Example 2(g) Example 3(g) Example 4(g) Topiramate Main drug 45 45 45 45 microcrystalline cellulose filler 50 50 50 50 hypromellose Adhesive 3 3 3 3 calcium carbonate stabilizer 3 3 3 3 Ethyl cellulose Coating material - 5.73 6.88 8.60 hypromellose Coating porogen - 2.45 2.94 3.68 Diethyl phthalate Coating plasticizer - 1.82 2.18 2.73
[0028] Embodiment 1-4 Sustained-release granule preparation process:
[0029] a. Add the prescription amount of topiramate, filler and stabilizer into the fluidized bed, preheat to 35°C, and then spray the binder hypromellose aqueous solution on the added material through the top spraying process, and granulate.
[0030] b. Sizing the drug-containing granules, collecting 250-550 μm particles and adding them to the fluidized bed, and performing ...
Embodiment 5-6
[0037] Embodiment 5-6 sustained-release granule prescription:
[0038] Material name effect Example 5(g) Example 6(g) Topiramate Main drug 45 45 microcrystalline cellulose filler 50 50 hypromellose Adhesive 3 3 calcium carbonate stabilizer - 1 Ethyl cellulose Coating material 5.73 5.73 hypromellose Coating porogen 2.45 2.45 Diethyl phthalate Coating plasticizer 1.82 1.82
[0039] Embodiment 5-6 Sustained-release granule preparation process:
[0040] a. Add the prescription amount of topiramate, filler, and stabilizer into the fluidized bed, preheat to 35°C, and then spray the binder hypromellose aqueous solution on the added material through the top spraying process, and granulate.
[0041] b. Sizing the drug-containing granules, collecting 250-550 μm particles and adding them to the fluidized bed, and performing slow-release coating on the drug-containing granules through the bottom spraying pro...
Embodiment 2、5、6
[0043] Embodiment 2, 5, 6 Influencing factors 10 days related substance (%) determination result
[0044]
[0045] From the above experimental results, it can be seen that the stabilizer has a protective effect on the sample and prevents the degradation of the drug, and the more the stabilizer is used, the more beneficial it is for the stability of the sample.
PUM
| Property | Measurement | Unit |
|---|---|---|
| melting point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com