A kind of cefaclor sustained-release composition and preparation method thereof
A technology of cefaclor and its composition, which is applied in the field of pharmaceutical preparation, can solve problems such as failure to maintain therapeutic blood drug concentration, delayed release of target drug release, failure to meet 4 hours, etc., to achieve improved compliance and high safety , the effect of reducing the frequency of administration
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
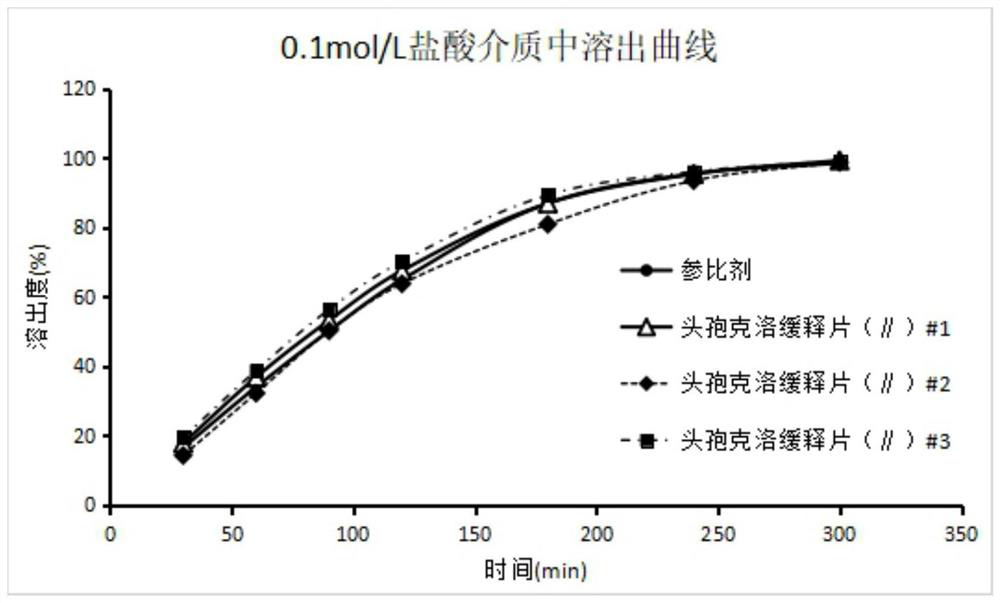
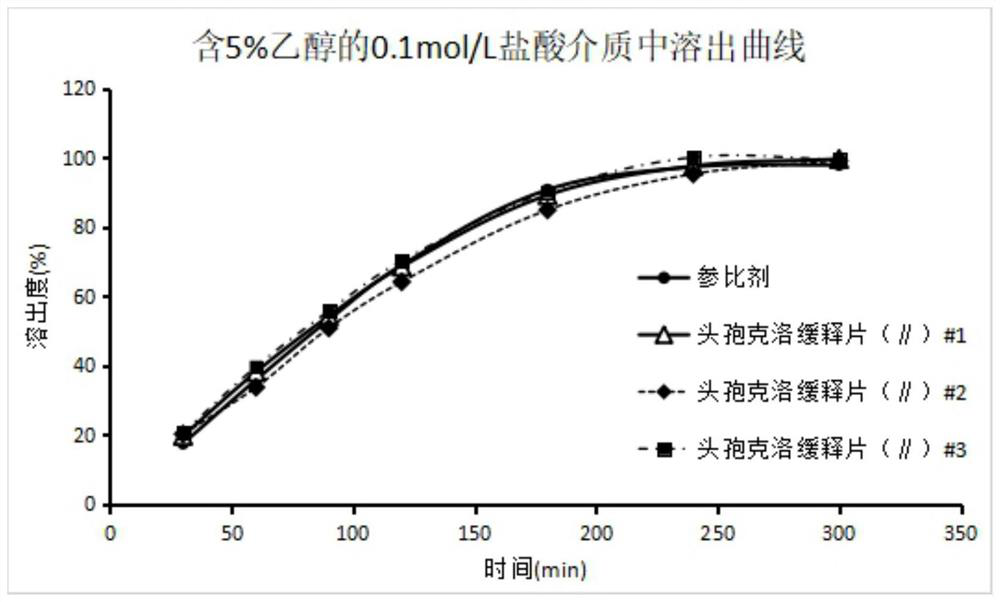
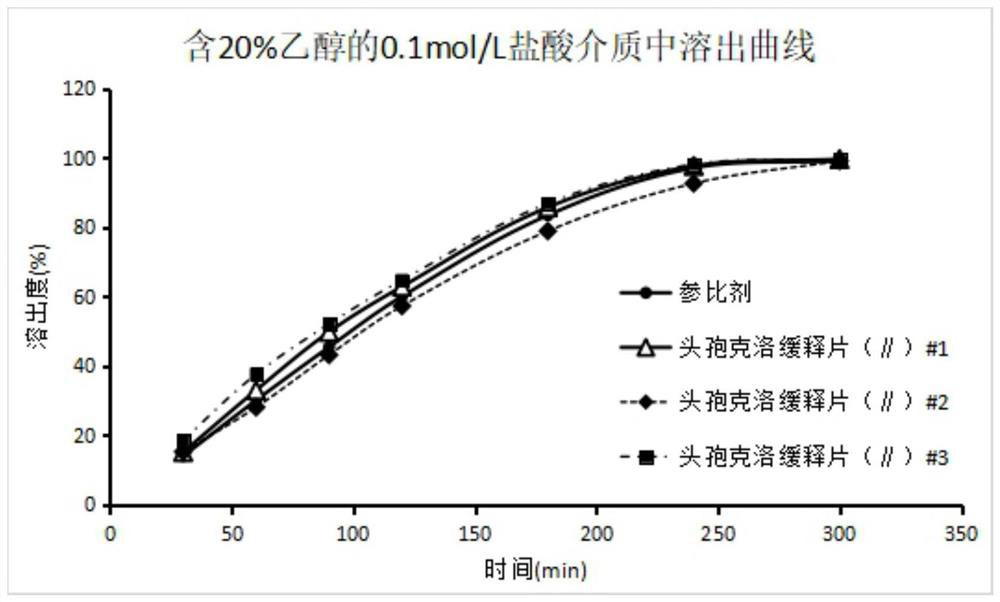
Image
Examples
Embodiment 1
[0048] Embodiment 1 Cefaclor sustained-release tablet (∥) #1 and preparation method thereof
[0049] The present embodiment provides a kind of cefaclor sustained-release tablet (∥) #1, is tablet, and every 1000 tablets content is as follows:
[0050] Element Content (g) Function Cefaclor 375 73.26% Mannitol 30 5.86% HPMC-E50 73.2 10.28% HPC-HXF 50 8.13% Udage L100-55 19.4 1.31% stearic acid 3.5 0.68% Magnesium stearate 2.5 0.49% Total piece weight 512 Opadry Blue Coating Powder 16 3.12%
[0051] The preparation method of the Cefaclor Sustained Release Tablet (∥) #1 includes: sieving raw and auxiliary materials, weighing and mixing, granulating, drying, tabletting, coating and other processes. The specific process steps are as follows:
[0052] Step 1: Sieve the cefaclor raw material, HPC-HXF, HPMC-E50, and mannitol separately, mix and store for later use;
[0053] Step 2: Dissolve in ethanol ac...
Embodiment 2
[0060] Embodiment 2 Cefaclor sustained release tablet (∥) #2 and preparation method thereof
[0061] The present embodiment provides a kind of cefaclor sustained-release tablet (∥) #2, is tablet, and every 1000 tablets content is as follows:
[0062] Element Content (g) Function Cefaclor 375 72.84% Mannitol 30 5.83% HPMC-E5 34 6.60% HPC-HXF 61.3 11.91% Udage L100-55 7.5 1.46% stearic acid 4 0.78% Magnesium stearate 3 0.58% Total piece weight 514.8 Opadry Blue Coating Powder 16 3.12%
[0063] The preparation method of the cefaclor sustained-release tablet (∥)#2 includes: sieving raw and auxiliary materials, weighing and mixing, granulating, drying, tableting, coating and other processes. The specific process steps are as follows:
[0064] Step 1: Sieve the cefaclor raw material, HPC-HXF, HPMC-E50, and mannitol separately, mix and store for later use;
[0065] Step 2: Dissolve in ethanol accordin...
Embodiment 3
[0072] Example 3 Cefaclor Sustained Release Tablet (∥) #3 and its preparation method
[0073] The present embodiment provides a kind of cefaclor sustained-release tablet (∥) #3, is tablet, and every 1000 tablets content is as follows:
[0074] Element Content (g) Function Cefaclor 375 72.74% Mannitol 30 5.82% HPMC-K100LV 76.6 14.86% HPMC-E50 21.5 4.17% Udage L100-55 7.1 1.38% stearic acid 3.2 0.62% Magnesium stearate 2.1 0.41% Total piece weight 515.5 Opadry Blue Coating Powder 16 3.12%
[0075] The preparation method of the cefaclor sustained-release tablet (∥)#3 includes: sieving raw and auxiliary materials, weighing and mixing, granulating, drying, tableting, coating and other processes. The specific process steps are as follows:
[0076] Step 1: Sieve the cefaclor raw material, HPC-HXF, HPMC-E50, and mannitol separately, mix and store for later use;
[0077] Step 2: Dissolve in ethanol acco...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com