Preparation method of functional hydrogel medical dressing
A manufacturing method and technology for hydrogels, which are applied in the fields of medical science, absorbent pads, bandages, etc., can solve the problems of fragility, increased toxicity of the gel system, and low mechanical strength of hydrogels, avoiding biological toxicity and achieving excellent dissolution. sex, and the effect of promoting the growth of epithelial cells
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
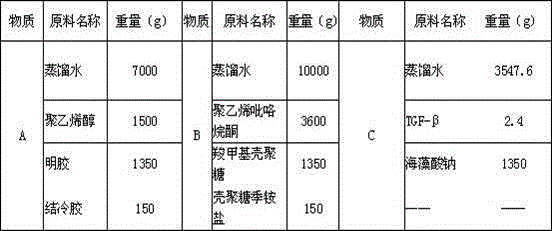
[0063] A 30Kg functional hydrogel medical dressing is composed of 1800g of biopolymer material, 3000g of chemically synthesized polymer material, 1.125g of transforming growth factor TGF-β and 25198.875g of distilled water, wherein the biopolymer material is composed of carboxymethyl Chitosan 450g, chitosan quaternary ammonium salt 450g, gelatin 450g, gellan gum 150g, sodium alginate 300g, chemically synthesized polymer material is composed of polyvinyl alcohol 1500g and polyvinylpyrrolidone 1500g.
[0064] The functional hydrogel medical dressing of the present embodiment 1 is made according to the following steps:
[0065] Step 1. Measure the components of Substance A, Substance B and Substance C according to Table 1 below, and blend them using the following steps to form a physically crosslinked hydrogel:
[0066]
[0067] Table 1
[0068] a. Put each component material of substance A in the above table 1 into a vacuum compounder and stir for 30 minutes, make the materi...
Embodiment 2
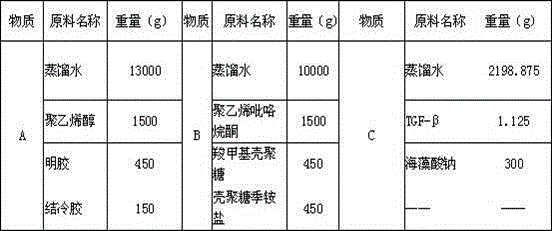
[0085] A 30Kg functional hydrogel medical dressing in the proportion of Example 2 is composed of 2100g of biopolymer material, 3900g of chemically synthesized polymer material, 1.125g of transforming growth factor TGF-β and 23998.875g of distilled water, wherein The molecular material is composed of 450g of carboxymethyl chitosan, 450g of chitosan quaternary ammonium salt, 450g of gelatin, 300g of gellan gum, and 450g of sodium alginate. The chemically synthesized polymer material is composed of 2400g of polyvinyl alcohol and 1500g of polyvinylpyrrolidone composition.
[0086] The functional hydrogel medical dressing of the present embodiment 2 is made according to the following steps:
[0087] Step 1. Measure each component of material A, material B and material C according to the following table 2, and use the following steps to blend them to form a physically cross-linked hydrogel:
[0088]
[0089] Table 2
[0090] a. Put each component material of Substance A in Tabl...
Embodiment 3
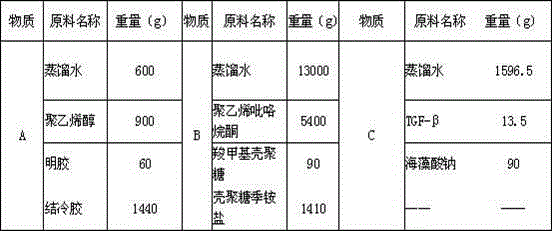
[0107] A 30Kg functional hydrogel medical dressing in the proportion of Example 3 is composed of 3090g of biopolymer material, 6300g of chemically synthesized polymer material, 13.5g of transforming growth factor TGF-β and 20596.5g of distilled water. The molecular material is composed of carboxymethyl chitosan 90g, chitosan quaternary ammonium salt 1410g, gelatin 60g, gellan gum 1440g, sodium alginate 90g, chemically synthesized polymer material is composed of polyvinyl alcohol 900g and polyvinylpyrrolidone 5400g composition.
[0108] The functional hydrogel medical dressing of present embodiment 3 is made according to the following steps:
[0109] Step 1. Measure each component of material A, material B and material C according to the following table 3, and use the following steps to blend them to form a physically cross-linked hydrogel:
[0110]
[0111] table 3
[0112] a. Put each component material of Substance A in the above Table 3 into a vacuum mixer and stir for...
PUM
| Property | Measurement | Unit |
|---|---|---|
| tensile strength | aaaaa | aaaaa |
| tensile strength | aaaaa | aaaaa |
| tensile strength | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com