Preparation method of quinacridone intermediate
A technology of quinacridone and intermediates, which is applied in the preparation of organic compounds, chemical instruments and methods, and cyanide reaction preparation, etc., which can solve the problems of large environmental pollution, high energy consumption, and large amounts of organic waste, and achieve reduction The effect of organic waste
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
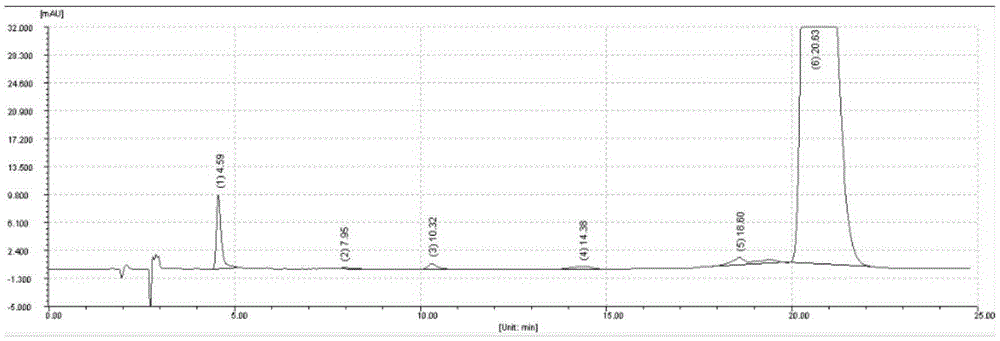
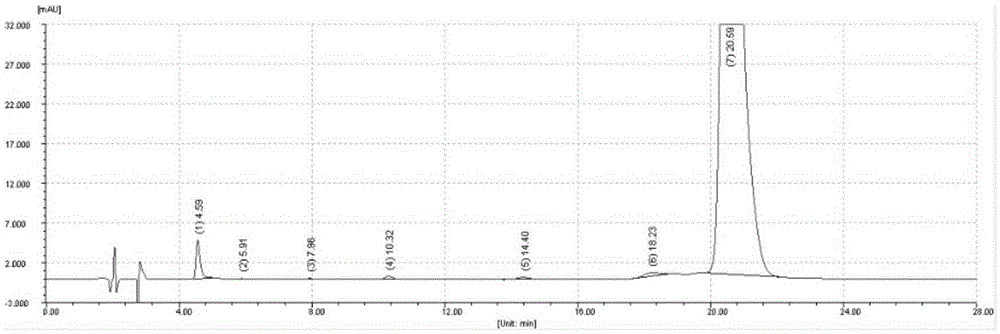
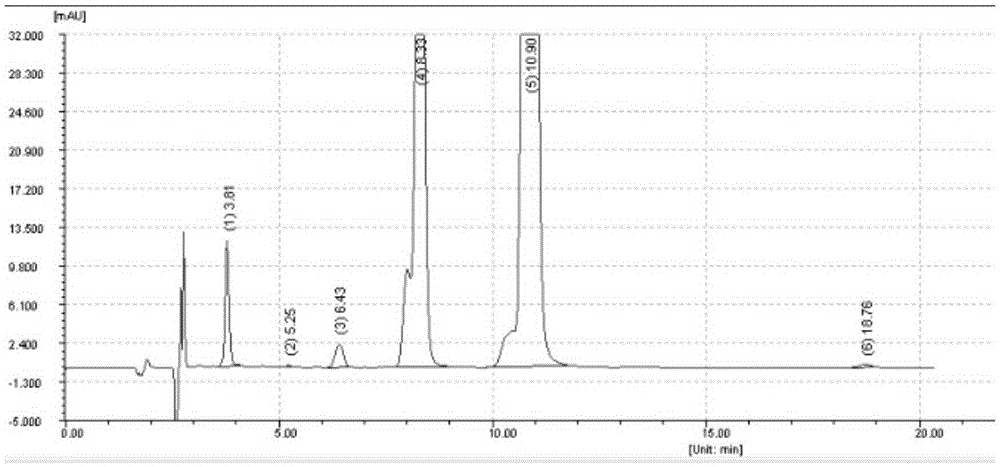
Image
Examples
Embodiment 1
[0060]Add 500 parts of methanol, 69.4 parts of p-toluidine, 70 parts of dimethyl succinyl succinate, 1.3 parts of hydrochloric acid (31%) and 0.6 parts of concentrated sulfuric acid (98%) in sequence, into 1500 parts of the reaction kettle, and stir For 20 minutes, heat up to reflux, reflux for 4 hours, cool down to 55°C, control at 50-60°C, add 0.7 parts of anthraquinone-2,6-sodium disulfonate, add 90 parts of 30% hydrogen peroxide in 60 minutes, and complete the addition Stir for 5 minutes, add 160 parts of 50% sodium hydroxide in 30 minutes, stir for 20 minutes after adding, heat up to reflux, reflux for 4 hours, cool down to 65°C, add 190 parts of water, stir for 10 minutes, add 1060 parts of water, filter, Collect the filtrate. Measure the pH value of the filtrate, acidify and precipitate with 15% hydrochloric acid, take 30-60 minutes, adjust the pH value to 2.0, stir for 30 minutes, filter, wash the filter cake with tap water, dry and pulverize to obtain the finished int...
Embodiment 2
[0067] Add 680 parts of ethanol, 95 parts of aniline, 87.3 parts of dimethyl succinyl succinate, 4 parts of hydrochloric acid (31%) and 4 parts of concentrated sulfuric acid into the autoclave successively, stir for 20 minutes, seal the autoclave, and heat up to 95°C, keep warm for 3 hours, cool down to 5-55°C, add 0.9 parts of anthraquinone-2,6-sodium disulfonate, add 150 parts of 30% hydrogen peroxide in 90 minutes, stir for 5 minutes, use at 50-60°C Add 210 parts of 50% potassium hydroxide in 30 minutes, stir for 20 minutes after adding, seal the autoclave, heat up to 95°C and keep it for 4 hours, cool down to 60°C, add 3500 parts of water, stir for 15 minutes, keep the temperature at about 35°C, filter , wash the filter residue with 350 parts of water, merge into the filtrate, collect the filtrate, adjust the pH value to 1.5 with 30% sulfuric acid, stir for 30 minutes, retest the pH value should be 1.5, filter, wash with tap water, dry and pulverize to obtain the intermedia...
Embodiment 3
[0073] Add 680 parts of ethanol, 20 parts of aniline, 85 parts of p-toluidine, 87.5 parts of dimethyl succinylate, 2 parts of 31% hydrochloric acid and 0.8 parts of concentrated sulfuric acid into the autoclave in sequence, stir for 20 minutes, and seal the pressure Kettle, heat up to 100°C, keep warm for 4 hours, cool down to 50-60°C, add 1.8 parts of anthraquinone-2,6-sodium disulfonate, add 150 parts of 30% hydrogen peroxide in 60 minutes, stir for 5 minutes, at 50- Add 210 parts of 50% potassium hydroxide in 30 minutes at 60°C, stir for 20 minutes, heat up to 100°C, keep warm for 3 hours, cool down to 60°C, add 3500 parts of water, the temperature is about 35°C, filter, and wash the filter residue with 350 parts of water , merged into the filtrate, collected the filtrate, adjusted the pH value to 1.5 with 31% hydrochloric acid, stirred for 30 minutes, retested the pH, filtered, washed with tap water, dried, and pulverized to obtain the intermediate product. 127 parts were ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com