Site-directed mutagenesis modified saccharomyces cerevisiae dipeptidyl peptidase III
A technology of dipeptidyl peptidase and site-directed mutagenesis, applied in the direction of peptidase, enzyme, enzyme, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0024] Example 1: Synthesis of wtyDPP and myDPP
[0025] The present invention takes the dipeptidyl peptidase gene (NCBI database NM_001183312) sequence of SaccharomycescerevisiaeS288c as a reference, and adds 5'-GTC at the 5' end GAATTC -3' join 3'- at the 3' end CCTAGG GAC-5', ( GAATTC ) is the restriction enzyme cutting site EcoRI, ( GGATCC ) is the restriction enzyme cutting site BamHI. The wtyDPP gene was synthesized by artificial total synthesis.
[0026] The present invention takes the dipeptidyl peptidase gene (NM_001183312) sequence of SaccharomycescerevisiaeS288c as a reference, the amino acid at position 570 is replaced with alanine (Alanine, ALa, A), and the amino acid at position 572 is replaced with lysine (Lysine, Lys , K) substitution, the amino acid at position 574 is replaced with histidine (Histidine, His, H), and 5'-GTC is added at the 5' end GAATTC -3' join 3'- at the 3' end CCTAGG GAC-5', ( GAATTC ) is the restriction enzyme cutting site Eco...
Embodiment 2
[0029] Example 2: Construction of recombinant plasmids wtyDPP and myDPP expression plasmids
[0030] Gene cloning was carried out according to conventional methods (Sambrook, et al. 2001, Molecular Cloning A Laboratory Manual. Cold Spring Harbor Laboratory Press. USA), and the wtyDPP and myDPP obtained in Example 1 were respectively cloned into pHIL-S1 to construct expression plasmids pHIL-S1-wtyDPP and expression plasmids pHIL-S1 -myDPP, the cloned target gene was identified by enzyme digestion and sequencing.
[0031] specific methods:
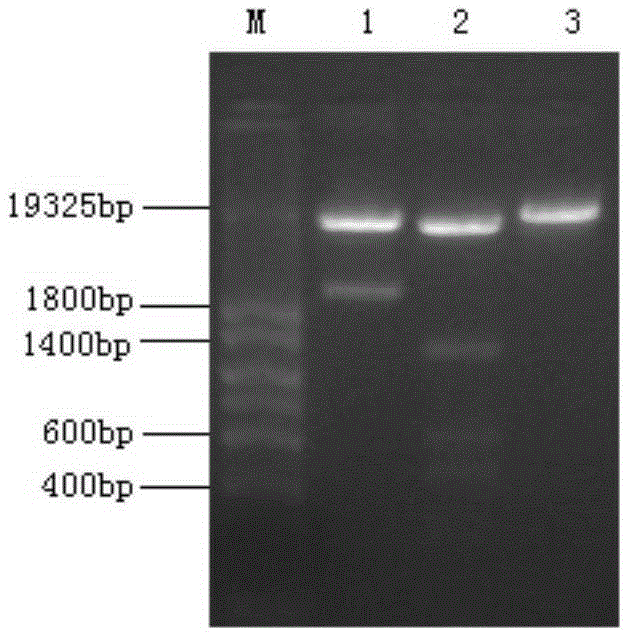
[0032] The construction process of the recombinant plasmid pHIL-S1 containing myDPP is as follows: EcoRI+BamHI double-digestion plasmid pHIL-S1 and the target fragment myDPP, and the digested product was subjected to 0.8% agarose gel electrophoresis, and the gel was cut and recovered. Ligation of plasmid pHIL-S1 and myDPP was performed using T4 DNA ligase. CaCl 2 Prepare E.coli DH5α competent cells, transform DH5α competent cells, screen ...
Embodiment 3
[0034] Example 3: Expression of recombinant myDPP and recombinant wtyDPP
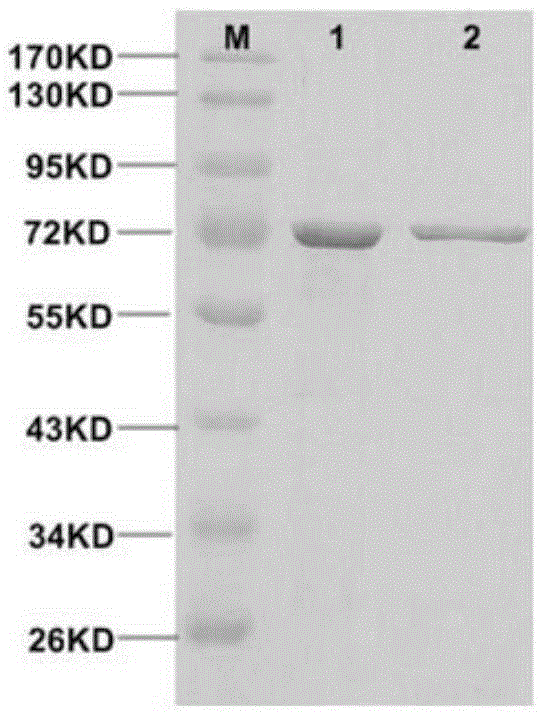
[0035] Expression of recombinant myDPP: Recombinant plasmid pHIL-S1-myDPP and plasmid pHIL-S1 were digested with SacI, the digested products were subjected to 0.8% agarose gel electrophoresis, and the linear recombinant plasmids pHIL-S1-myDPP and Plasmid pHIL-S1. Transform Pichia yeast GS115 according to the protoplast method in the PichiaExpressionKit (InvitrogenInc., U.S.) manual, and screen Mut + Turn. In the induced expression of recombinant bacteria using methanol as the sole carbon source (operated according to the PichiaExpressionKit manual), the SDS-PAGE electrophoresis analysis of the culture solution showed that after the transformant was induced to express, the supernatant of the culture solution showed an obvious target protein band, and the transformation The control bacteria without the empty plasmid containing the target gene were induced under the same conditions for 96 hours, and no t...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com