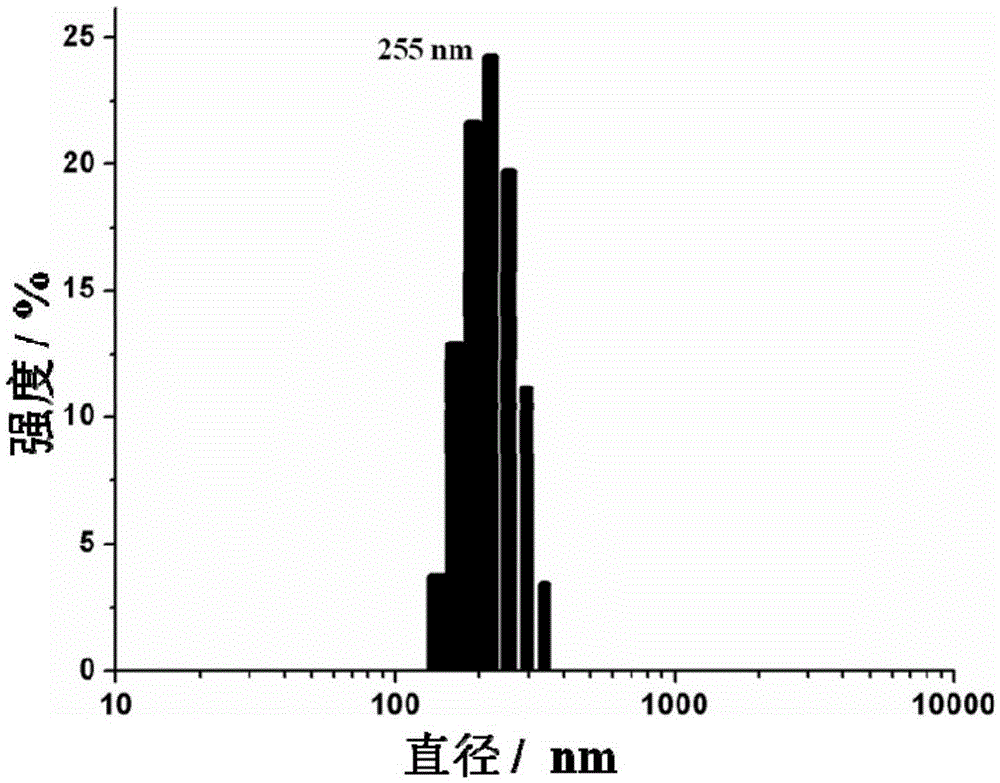
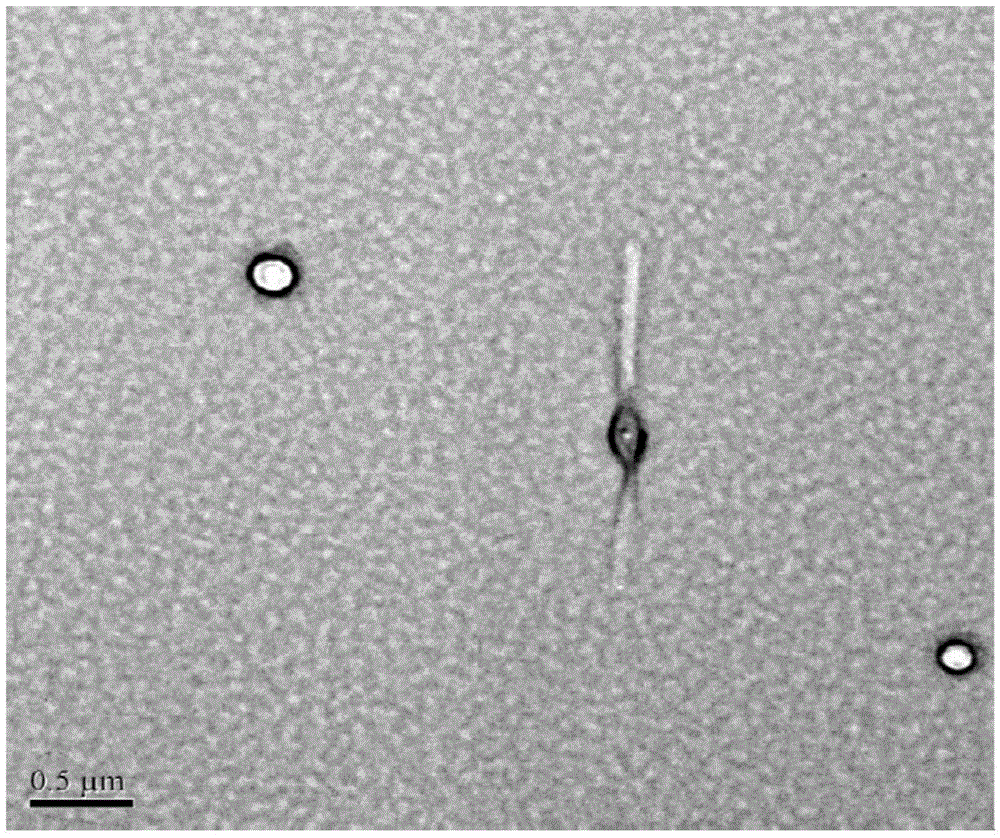
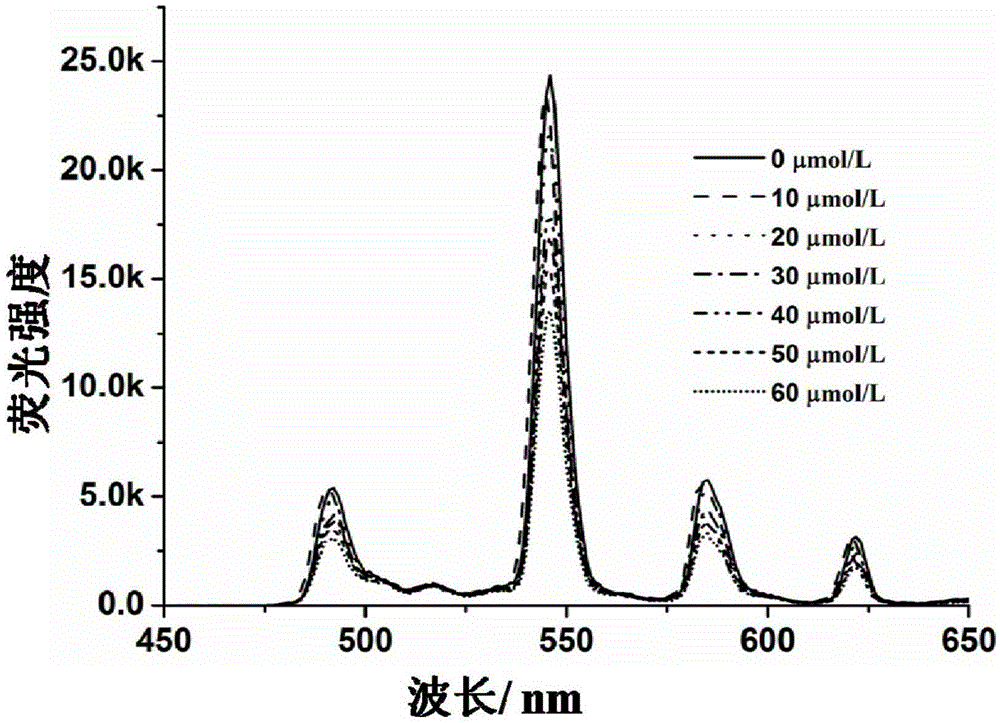
Amphiphilicity guanosine derivative, and preparation method therefor and application thereof in cytidine triphosphate sensing and recognition
A cytidine triphosphate and amphiphilic technology, which is applied in the preparation of sugar derivatives, sugar derivatives, sugar derivatives, etc., can solve the problems that limit the practical application of chemical sensors, achieve selective recognition, mild reaction conditions, The effect of simple preparation method
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0042] 1. Synthetic formula I compound
[0043] Dissolve 3.5g (12mmol) of guanosine in 140mL of acetone, then add 2.34g (12mmol) of p-toluenesulfonic acid (TsOH), 35mL (284mmol) of 2,2-methoxypropane, and stir at room temperature for 24 hours. After the reaction was over, the acetone was distilled off under reduced pressure, and 25 mL of distilled water was added to stir and 1.038 g of NaHCO was added in batches. 3 solid, stirred at room temperature for 2 hours, then added 25 mL of saturated NaHCO dropwise 3 The aqueous solution was stirred for 2 hours, suction filtered, and then washed with water. After repeating 2 to 3 times, the obtained solid was vacuum-dried to obtain a white powdery solid, which was the compound of formula I. The reaction equation was as follows:
[0044]
[0045] The structural characterization data of the compound of formula I obtained is: 1 H-NMR (600MHz, DMSO, Me 4 Si): 10.69 (1H, NH), 7.92 (1H, CHN), 6.52 (2H, NH 2 ), 5.93 (1H, CHN), 5.18 (1H...
Embodiment 2
[0059] The use of the amphiphilic guanosine derivatives of Example 1 in the sensing and recognition of cytidine triphosphate, the specific method is as follows:
[0060] 1. Add the amphiphilic Tb(III) complex into 10mmol / L 4-hydroxyethylpiperazineethanesulfonic acid buffer solution with a pH value of 7.4, keep it warm at 50-60°C for 1-3 hours, and cool naturally to room temperature , standing at room temperature for 8-12 hours to obtain a 100 μmol / L amphiphilic Tb(III) complex solution.
[0061] The structural formula of the above-mentioned amphiphilic Tb(III) complex is as follows:
[0062]
[0063] 2. Add amphiphilic guanosine derivatives to 10mmol / L 4-hydroxyethylpiperazineethanesulfonic acid buffer solution with a pH value of 7.4, keep warm at 50-60°C for 1-3 hours, and cool naturally to room temperature. Stand still for 8-12 hours to obtain a 200 μmol / L amphiphilic guanosine derivative solution.
[0064] 3. Mix the 100 μmol / L amphiphilic Tb(III) complex solution obta...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com