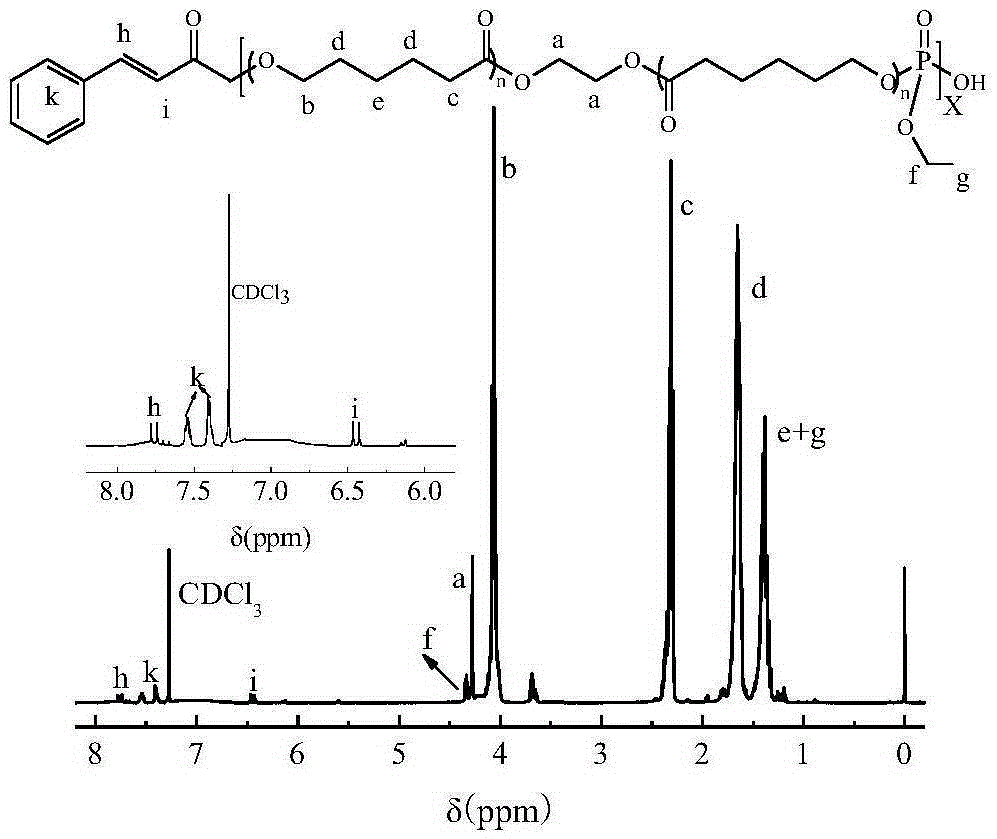
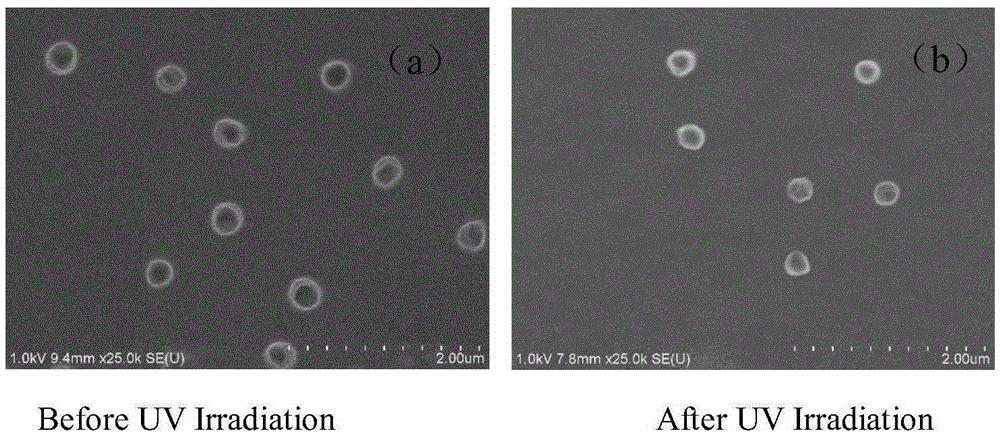
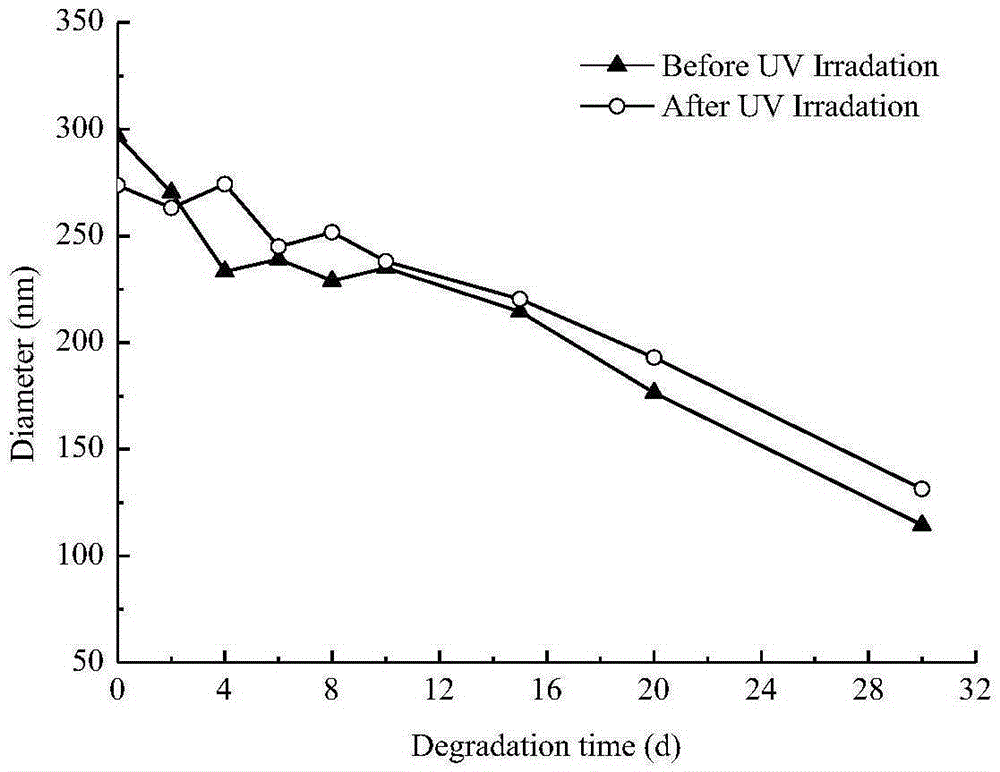
Degradable photosensitive phosphate copolymers
A phosphate-based, photosensitive technology, applied in the field of functional polymer materials, can solve the problems of low melting temperature and glass transition temperature, less phosphate polymer, poor hydrophilicity, etc., and achieve good biodegradability and biocompatibility capacitive effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0012] Embodiment 1, poly (ε-caprolactone) (PCL)
[0013] According to the feeding ratio of 1:6, the initiator EG (0.6206, 10mmol), the monomer ε-CL (6.8412, 60mmol) and the solvent toluene (50mL) were added to a 100mL three-necked flask respectively, at 100 ° C, N 2 After stirring for 30 minutes under the atmosphere, add a certain amount of catalyst stannous octoate (Sn(Oct) 2 ), continue to react with stirring at 100°C for 24 hours. After the reaction, the toluene solution of the product was added dropwise into anhydrous ether at a certain rate, precipitated, filtered with suction, and then dried in a vacuum oven at 30°C until constant weight.
Embodiment 2
[0014] Embodiment 2, phosphate ester polymer (PPE-PCL)
[0015] The pre-synthesized molecular weight is 1.70×10 3 The two-terminal hydroxyl PCL (11.2g, 10mmol) was placed in a three-necked flask, heated to 80°C to melt, and nitrogen gas was passed to remove the oxygen in the system. The equivalent of EDP (1.63g, 10mmol) was added dropwise, the system was sealed, vacuumized again and the temperature was gradually raised to 160°C, and the reaction was kept for 24h under a certain degree of vacuum, and then cooled to room temperature, and the product was dissolved with dichloromethane and used without Precipitate with diethyl ether, filter, and dry in a vacuum oven at 40°C.
Embodiment 3
[0016] Embodiment 3, photosensitive phosphate ester polymer (PPE-PCL-C)
[0017] PPE-PCL (4.11g, 1mmol), 25mL of dichloromethane and 1mL of pyridine were added to a 50mL round-bottomed flask, stirred at 0°C for 0.5 hours, 10 equivalents of cinnamoyl chloride (1.665g, 10mmol) was added, and After stirring and reacting for 1.5 h, the stirring reaction was continued at room temperature for 24 h. After the reaction, precipitate and filter with anhydrous ether for 3 times, wash the precipitate with ether until the filtrate has a completely transparent color, then wash with deionized water to remove pyridine hydrochloride and excess pyridine, and finally place it in a vacuum oven for 40 The final product was obtained by drying at °C.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com