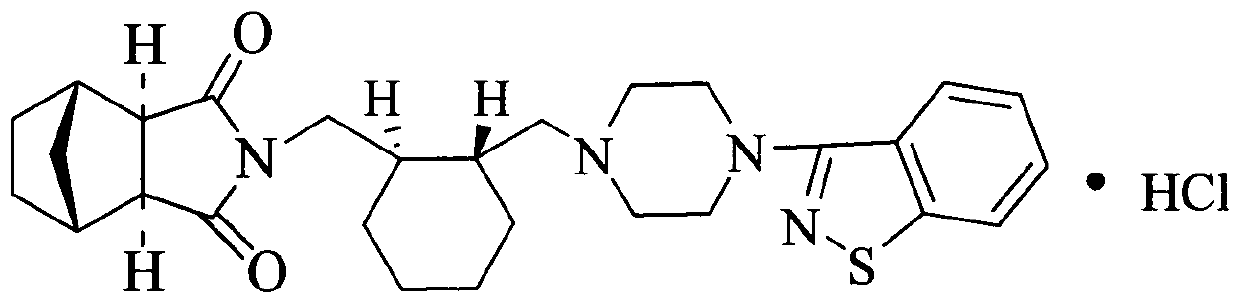
A kind of lurasidone hydrochloride tablet
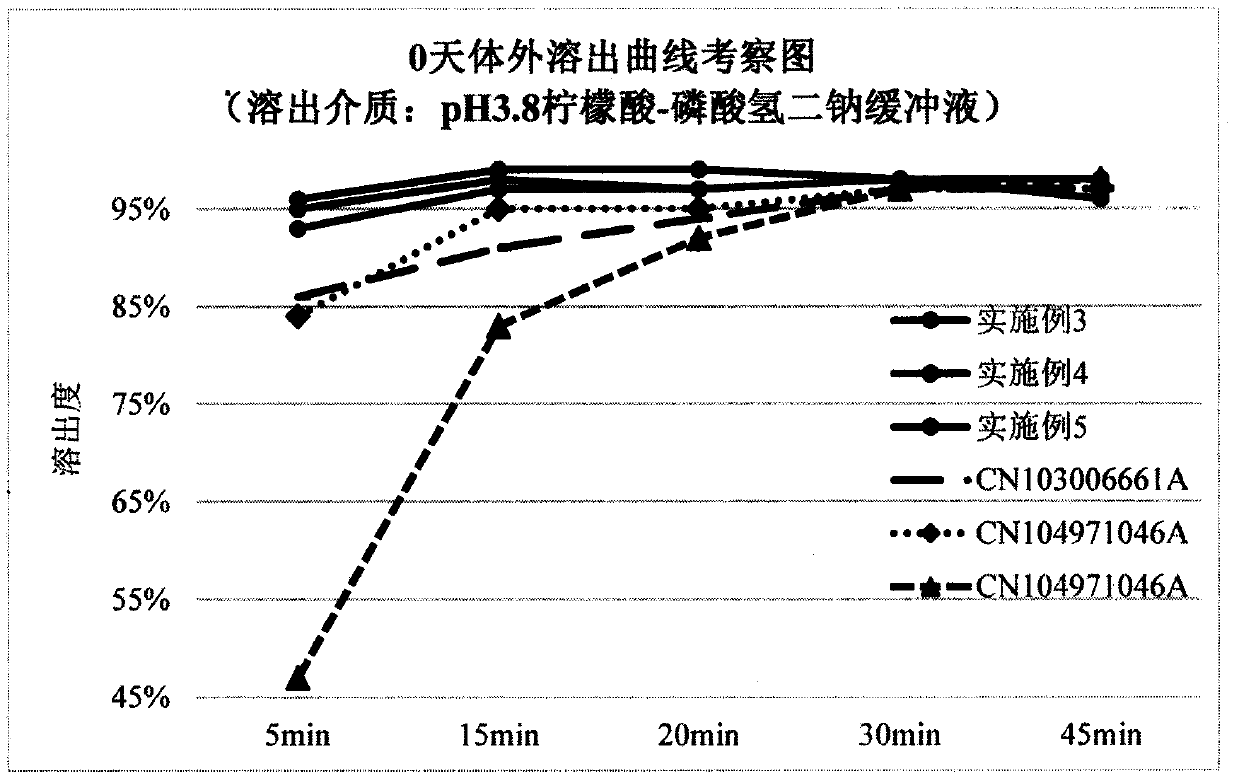
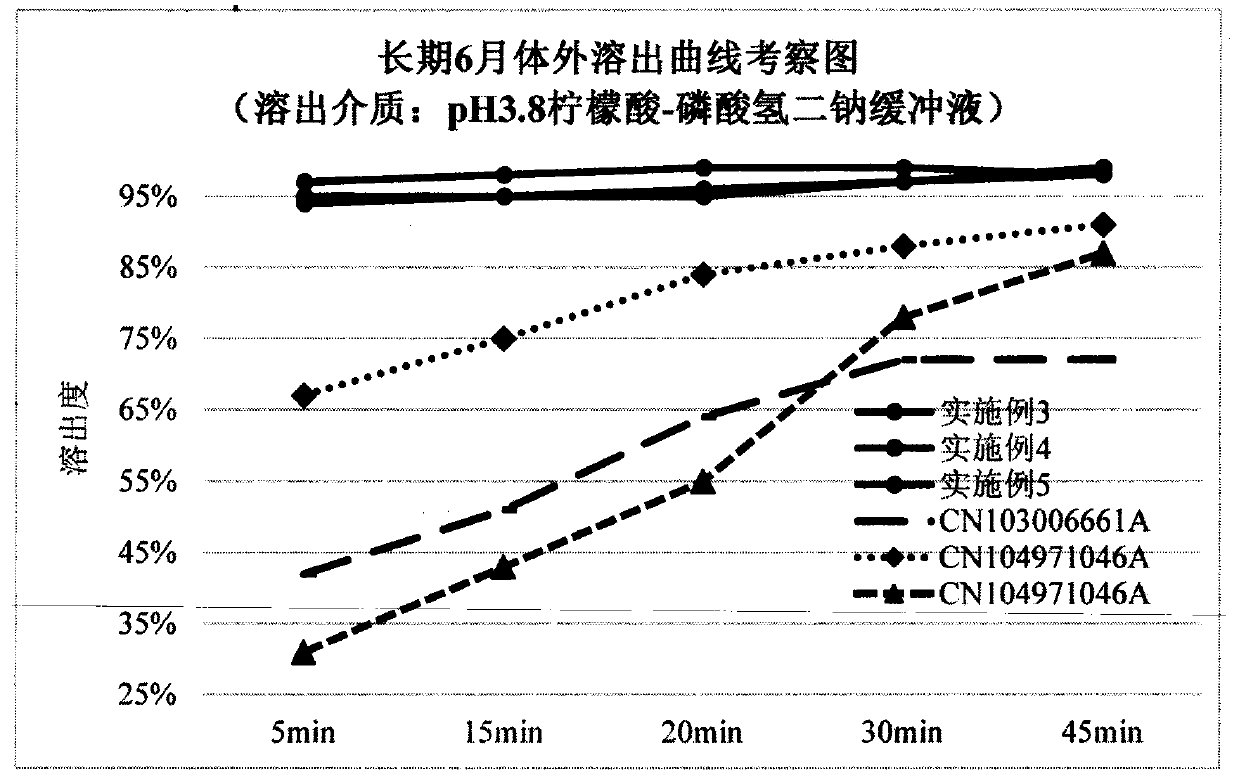
A technology of lurasidone hydrochloride tablets and lurasidone hydrochloride, which can be applied in the directions of pill delivery, nervous system diseases, drug combination, etc. The problems such as the decrease of the dissolution rate of ketone and the failure to prevent the decrease of the dissolution rate of lurasidone hydrochloride have achieved the effect of stable in vitro dissolution curve, no decrease in in vitro dissolution rate, and small batch difference
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0024] prescription:
[0025]
[0026] Preparation method: heat and melt potassium citrate and sorbitol in a hot-melt extruder, then add lurasidone hydrochloride to melt, extrude the melt to granulate, and mix with calcium hydrogen phosphate, micropowder silica gel, magnesium stearate direct compression
Embodiment 2
[0028] prescription:
[0029]
[0030] Preparation method: heat and melt potassium citrate and sorbitol in a hot-melt extruder, then add lurasidone hydrochloride to melt, extrude the melt to granulate, and mix with calcium hydrogen phosphate, micropowder silica gel, magnesium stearate Made directly into tablets.
Embodiment 3
[0032] prescription:
[0033]
[0034]
[0035] Preparation method: heat and melt potassium citrate and sorbitol in a hot-melt extruder, then add lurasidone hydrochloride to melt, extrude the melt to granulate, and mix with calcium hydrogen phosphate, micropowder silica gel, magnesium stearate Made directly into tablets.
PUM
| Property | Measurement | Unit |
|---|---|---|
| volume | aaaaa | aaaaa |
| particle diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com