Cosmetics based on phaffia rhodozyma raw materials and preparation method thereof
A technology of Phaffia rhodozyme and cosmetics, applied in the field of cosmetics and its preparation based on Phaffia rhodozyme raw materials, achieving high safety effects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
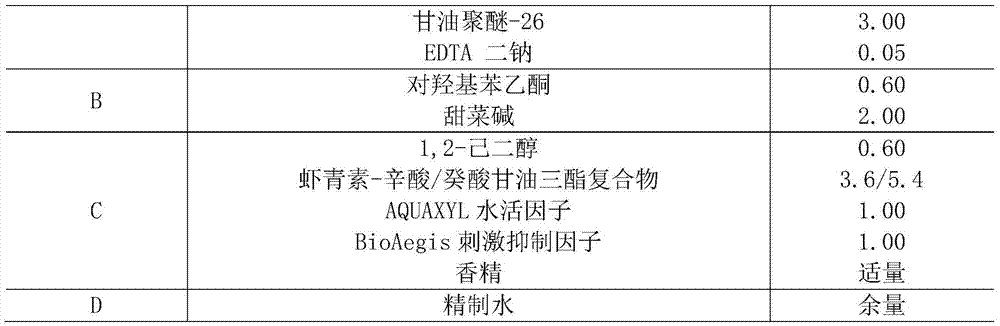
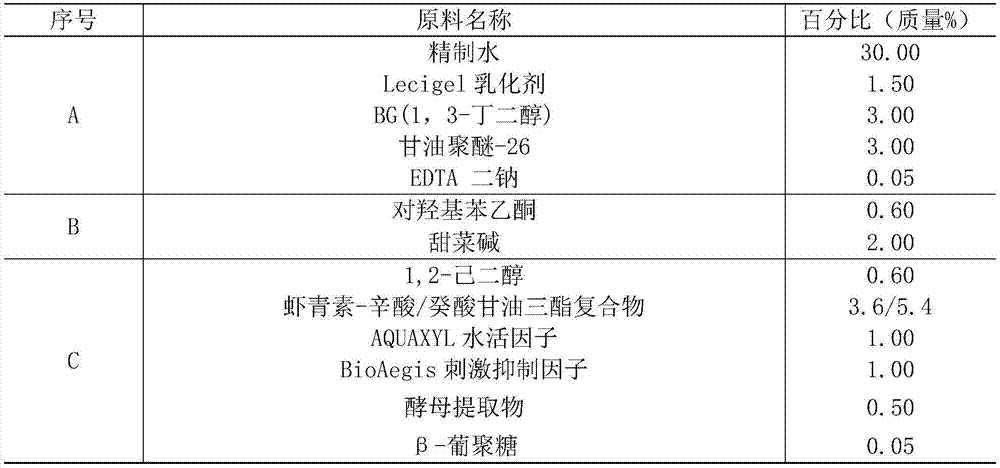
[0082] (1) The volume of the reaction system is 1L, containing 100g of Phaffia rhodozyma (ATCC66270) cells and 60mL of cellulase, the pH value is adjusted to about 4.5 with 3M hydrochloric acid, and the volume is constant with deionized water; React at ℃ for 6h, boil for 3min to inactivate the enzyme activity.
[0083] (2) After the reaction, centrifuge at 10,000 rpm for 10 min to wash the precipitate.
[0084] (3) Add 60 mL / g of edible ethanol to the yeast after enzymatic hydrolysis, shake and extract in the dark for 60 minutes on a shaking table with a rotation speed of 150 rpm, centrifuge at 10000 rpm for 10 minutes, collect the supernatant, and obtain an astaxanthin extraction rate of 91.93% (by The content of astaxanthin was determined by spectrophotometer, and the amount of astaxanthin extracted by the acid-heat treatment method was used as a contrast, that is, the astaxanthin extracted by the default hot acid method was the total amount of astaxanthin, and the extractio...
Embodiment 2
[0088] Screening of any component of the astaxanthin complex of embodiment 2
[0089] (1) Take 20g caprylic / capric triglyceride, 20g isononyl isononanoate, 20g isopropyl myristate, 20g olive oil, 20g squalane, 20g sweet almond oil and 20g glycerin.
[0090] (2) Add the carotenoids prepared in Example 1 to the oils in step (1) with the amount of astaxanthin being 6% (mass fraction, ie 1.2g) Mix by a high-speed, high-shear mixer in a dark environment.
[0091] (3) centrifuge at 5000rpm for 10min, carry out solid-liquid separation, take the liquid, and obtain the complex of astaxanthin.
[0092] (4) The absorbance of each astaxanthin-oil complex at 474 nm was measured by spectrophotometry to determine the solubility of each oil component to the pigment. Among them, caprylic acid / capric triglyceride has the largest dissolution of pigment, OD 474 =292.35; followed by isopropyl myristate, OD 474 =57.4; then isononyl isononanoate, OD 474 =19.52; the rest of the oils dissolve les...
Embodiment 3
[0094] Example 3 Determination of DPPH free radical scavenging ability of astaxanthin-caprylic acid / capric triglyceride complex
[0095] (1) Dilute the 539.4 mg / L astaxanthin-caprylic acid / capric triglyceride complex mother liquor with absolute ethanol until the astaxanthin content is 5.4, 7.2, 9.6, 12.8, 17.1, 22.76, 30.34, 40.46, 53.94mg / L.
[0096] (2) The DPPH free radical ability of the astaxanthin complex at each concentration was determined by the DPPH method for scavenging organic free radicals (Anarjan N, et al. Colloidalastaxanthin: Preparation, characterization and bioavailability evaluation, Food Chem. 2012, 135(3): 1303-1309.). Obtain the linear relationship between the content of astaxanthin and its DPPH free radical scavenging rate in this concentration range, the relational formula is: Y=1.397x+5.585, R 2 =0.992, wherein Y is DPPH free radical scavenging rate (%), X is astaxanthin concentration (mg / L). It can be known that the IC of astaxanthin-caprylic acid / ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com