New monoterpene compound and preparation method and application thereof
A technology of compounds and compound structural formulas, applied in the field of new monoterpenoids and their preparation, to achieve precise pharmacological effects, simple extraction and separation methods, and complex chemical components
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
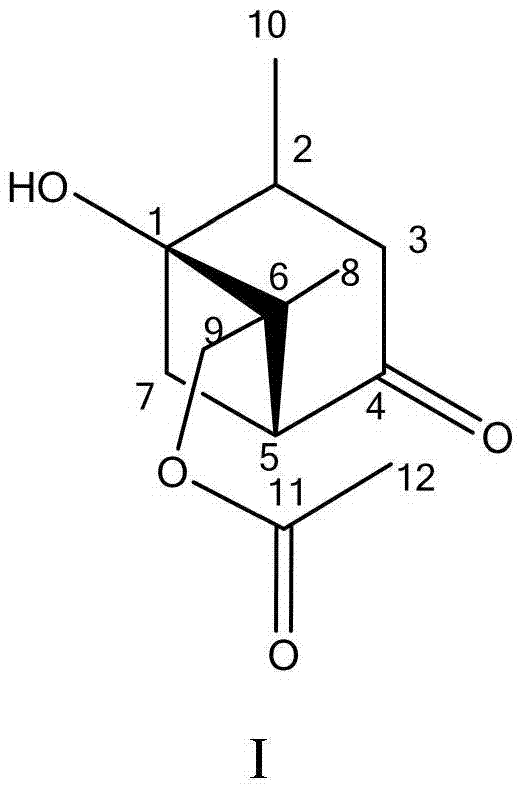
[0023] Take the dry root bark of Tibetan peony, crush it and accurately weigh 10kg, add 95% ethanol according to the ratio of root bark to solvent 1:10 (W / V), extract at room temperature for 24 hours, filter with suction, repeat three times, 50 °C and concentrated under reduced pressure, then the total extract was extracted with ethyl acetate, and the extract was concentrated under reduced pressure at 50 °C to obtain 356 g of component dry powder A.
[0024] Weigh 356g of dry powder A and mix it with 1.5kg of polyamide, carry out medium-pressure liquid chromatography, the reverse phase column packing material is MCI-Gel, and use 40% volume concentration of methanol aqueous solution to elute and elute to obtain 57g of component B, Dissolve in 120mL of methanol, mix the sample with silica gel, and perform silica gel column chromatography after the solvent is completely volatilized, and elute with a mixture of petroleum ether / isopropanol with a volume ratio of 10:1 to obtain compo...
Embodiment 2
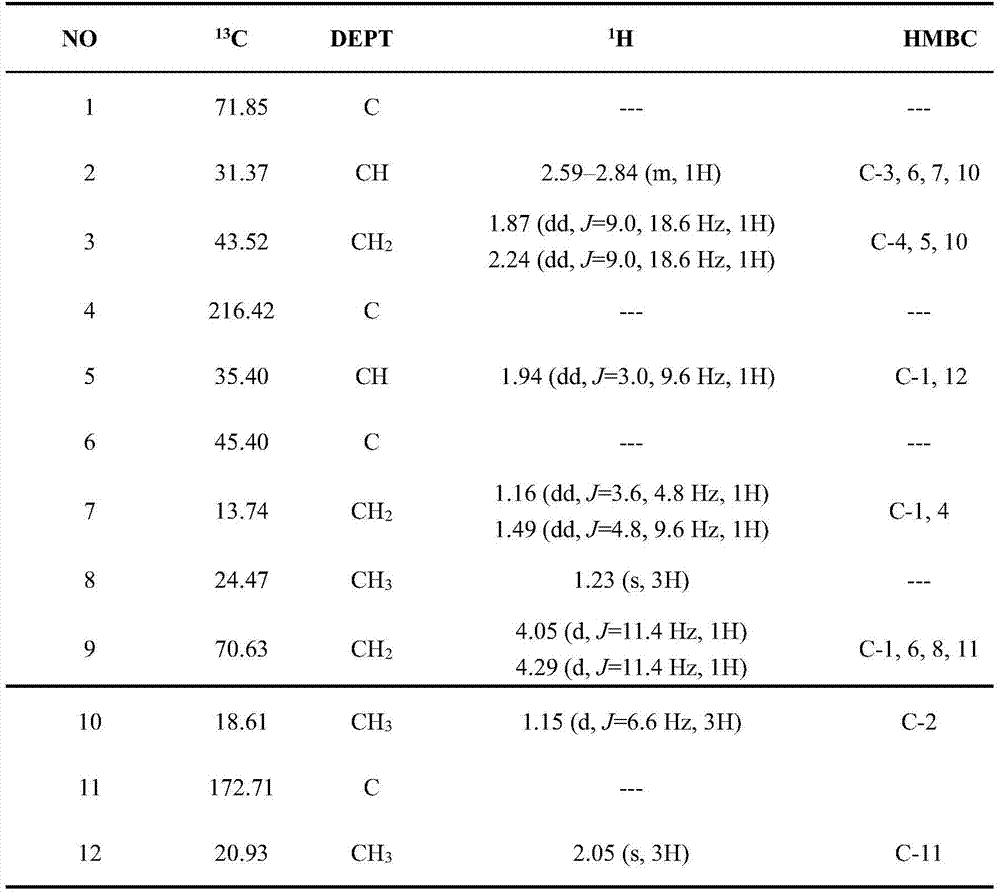
[0025] Embodiment 2 monomer compound structure identification
[0026] 1) Spectrum test
[0027] The isolated compound was tested for purity by analytical liquid chromatography, and if there was no ultraviolet absorption, it could not be detected by analytical liquid chromatography, and was tested by TLC. According to the polarity of the monomer compound, a corresponding deuterated solvent was selected for dissolution, and NMR was used for detection, and tetramethylsilane (TMS) was used as an internal standard. New compounds need to be pressed into KBr tablets for IR identification of the functional group types contained in the compound structure; at the same time, an appropriate amount of compound is taken and dissolved in a corresponding polar solvent for UV detection to determine the type of functional group with UV absorption in the compound structure. The molecular weight of the compound is estimated according to ESI-MS, and the molecular weight, molecular formula and de...
Embodiment 3
[0038] Example 3 Determination of antifungal activity
[0039] Microsporum gypsumoidis is a kind of filamentous fungus that can cause superficial skin ringworm, which can cause tinea corporis, jock itch, onychomycosis, tinea capitis, etc. It is a common superficial pathogenic fungus in clinical practice. In recent years, the role of medicinal plants in the treatment of skin diseases has attracted much attention. It is an important research direction to seek and use the active ingredients in natural medicinal plants to design new non-toxic drugs for the treatment of skin diseases. The minimum inhibitory concentration (Minimum inhibitory concentration, MIC) is an important index to measure the activity of antibacterial drugs. The test tube drug base method can not only conveniently interpret the MIC value, but also is simple, economical and easy to operate.
[0040] In this experiment, the activity of the isolated monomeric compounds was studied with reference to the "Spore-form...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com