Preparation method and application of D-dencichine
A technology of notoginseng and trifluoroacetoxy, which is applied in the fields of medicinal chemistry and therapeutics, can solve problems such as ineffective effects, and achieve good effects in treating thrombocytopenia, simple preparation process, and high yield.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
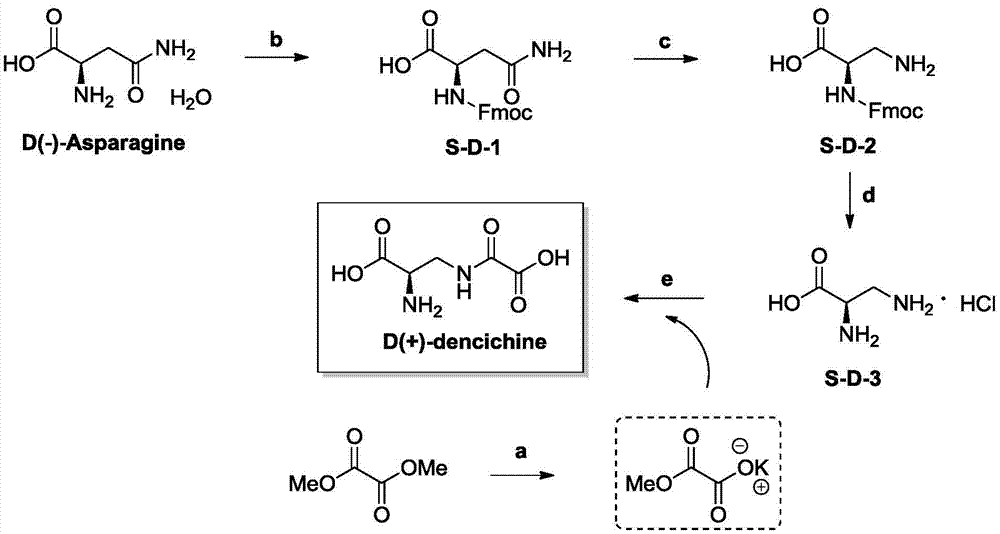
[0032] The preparation method of D-notoginseng provided by the invention comprises the following steps:
[0033] 1) performing Fmoc protection on the amino group of D-asparagine to obtain the first intermediate S-D-1;
[0034] 2) performing a Hoffman degradation reaction on the first intermediate S-D-1 to obtain a second intermediate S-D-2;
[0035] 3) under the action of an organic base, remove the Fmoc protection of the second intermediate S-D-2 to obtain the third intermediate S-D-3;
[0036] 4) Condensation reaction of the third intermediate S-D-2 with monopotassium oxalate under strong alkali conditions to obtain the target product D-notoginseng.
[0037] The synthetic route of D-notoginseng is as follows:
[0038]
Embodiment 1D- 10
[0039] The synthetic method of embodiment 1D-notoginseng
[0040] 1) Synthesis of the first intermediate S-D-1:
[0041] Take D-asparagine monohydrate (8.7g, 58mmol) in a round bottom flask, add distilled water 300mL, then add sodium bicarbonate (10.9g, 130mmol) and stir well; take 9-fluorenylmethyl-N-succinyl Imidocarbonate (20g, 60mmol) was dissolved in 150mL of acetone, and the acetone solution of the 9-fluorenylmethyl-N-succinimidylcarbonate was slowly added dropwise to the above-mentioned round-bottomed flask. The reaction was stirred for 4 hours; the pH value of the product solution was adjusted to 3 with concentrated hydrochloric acid, and then the product solution was filtered with suction to obtain 19.5 g of white solid, namely the first intermediate S-D-1, with a yield of 95%.
[0042] 2) Synthesis of the second intermediate S-D-2:
[0043] Take (24.9g, 56mmol) [bis(trifluoroacetoxy)iodo]benzene (PIFA) in a round bottom flask, add 58mL of acetonitrile to dissolve; ...
Embodiment 2
[0053] 1) Synthesis of the first intermediate S-D-1:
[0054] Take D-asparagine monohydrate (9.0g, 60mmol) in a round bottom flask, add distilled water 310mL, then add sodium bicarbonate (10.911.2g, 134mmol) and stir well; take 9-fluorenylmethyl-N-succinate Imidocarbonate (21g, 62mmol) was dissolved in 155mL of acetone, and the acetone solution of 9-fluorenylmethyl-N-succinimidylcarbonate was slowly added dropwise to the above-mentioned round-bottomed flask. The reaction was stirred for 3.5 hours; the pH value of the product solution was adjusted to 2.5 with concentrated hydrochloric acid, and then the product solution was filtered with suction to obtain 19.8 g of white solid, namely the first intermediate S-D-1, with a yield of 93%.
[0055] 2) Synthesis of the second intermediate S-D-2:
[0056] Take (24.9g, 56mmol) [bis(trifluoroacetoxy)iodo]benzene (PIFA) in a round bottom flask, add 58mL of acetonitrile to dissolve; take another (19.2g, 54.2mmol) S-D-1, dissolve in 115mL...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com