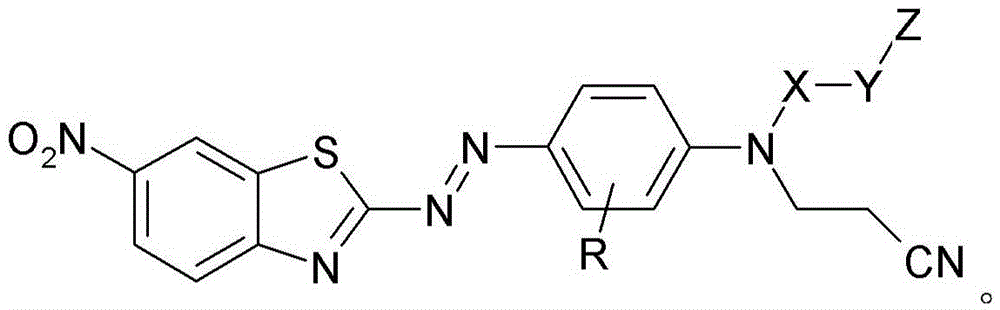
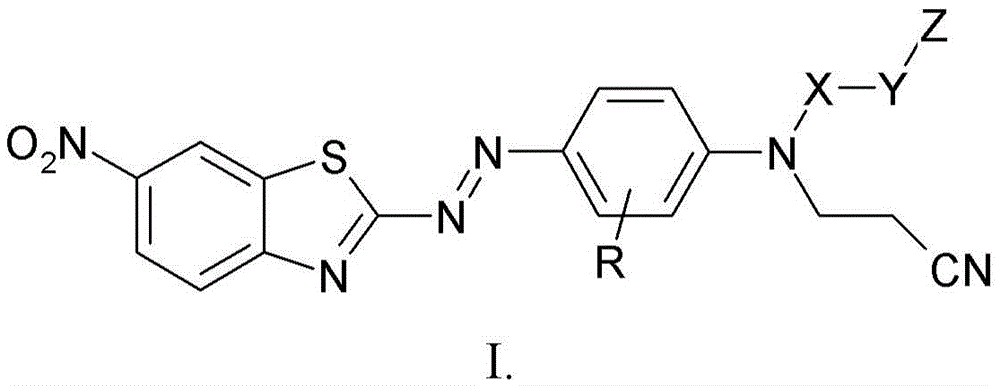
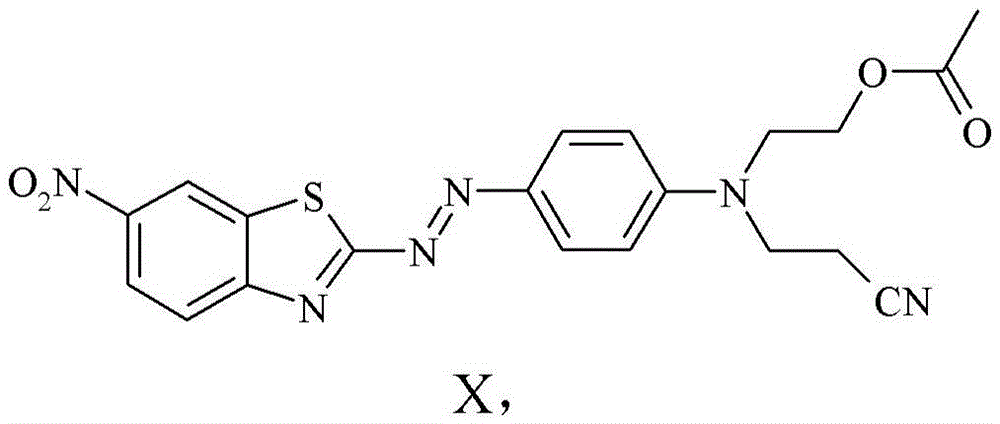
Benzothiazol-2-ylazo-phenyl compound as dye, compositions including the dye, and method of determining degree of cure of such compositions
A kind of technology of compound, composition, be used in benzothiazol-2-ylazo-phenyl compound as dyestuff
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
example
[0097] Reagent .
[0098] Vinyldimethylazlactone is commercially available from IsoChemSAS, Evry, France (IsoChem S.A.S., Evry, France). All other reagents were obtained or are commercially available from fine chemical suppliers such as: Sigma-Aldrich Company, St. Louis, Missouri; EMD Millipore Chemicals, Billerica, Mass. (EMDMillipore Chemicals, Billerica, Massachusetts); Alfa Aesar, Ward Hill, Massachusetts; J.T. Baker, Phillipsburg, New Jersey; Dorset, UK BDH Merck Ltd., Poole, Dorset, Uk, and Cambridge Isotope Laboratories, Inc., Andover, Massachusetts, or may be synthesized by known methods. All ratios are by weight unless otherwise reported.
[0099] The following abbreviations are used to describe instances:
[0100] ℃: degrees Celsius
[0101] cm: centimeter
[0102] CDCl 3 : deuterated chloroform
[0103] d 6 -DMSO: deuterated dimethyl sulfoxide
[0104] mg: milligram
[0105] mil: 10 -3 inch
[0106] mL: milliliter
[0107] mm: mm
[0108] mmol: milli...
example 1
[0117] Synthesis of 2-{(2-cyano-ethyl)-[4-(6-nitro-benzothiazol-2-ylazo)-phenyl]-amino}-ethyl 2-methacrylic acid ester :
[0118]
[0119] At about 21°C, 0.29 mL (2.1 mmol) of triethylamine was added to a 50 mL flask containing 0.55 g (1.39 mmol) of 3-{(2-hydroxy-ethyl)-[4-6-nitrate yl-benzothiazol-2-ylazo)-phenyl]-amino}-propionitrile in 20 mL of tetrahydrofuran, which was then cooled to 0°C. Then 162 μL (1.67 mmol) of methacryloyl chloride was added, and the mixture was stirred under a nitrogen atmosphere for 16 hours while maintaining the temperature at 0°C. The reaction mixture was filtered, and the filtrate was concentrated on a rotary evaporator. The resulting purple substance was dissolved in chloroform, washed twice with saturated sodium carbonate solution, twice with deionized water, and once with saturated sodium chloride solution. The organic portion was then dried over a bed of anhydrous sodium sulfate, filtered and concentrated on a rotary evaporator. The r...
example 2
[0121] Synthesis of 2-{(2-cyano-ethyl)-[4-(6-nitro-benzothiazol-2-ylazo)-phenyl]-amino}-ethyl acrylate:
[0122]
[0123] At about 21°C, 422 μL (3.03 mmol) of triethylamine was added to a 100 mL flask containing 0.399 g (1.01 mmol) of 3-{(2-hydroxyl-ethyl)-[4-(6- A solution of nitro-benzothiazol-2-ylazo)-phenyl]-amino}-propionitrile in 20 mL of N,N-dimethylformamide. This solution was stirred at about 21 °C for 10 minutes under nitrogen atmosphere. Then 195 μL (2.41 mmol) of acryloyl chloride was added. The flask was placed in an oil bath, and the mixture was stirred under a nitrogen atmosphere for 18 hours while maintaining the temperature at about 70°C. The reaction mixture was then partitioned between water (about 50 mL) and dichloromethane (about 50 mL). The aqueous layer was made basic by adding 5 mL of saturated aqueous sodium bicarbonate. The organic layer was then removed, and the aqueous layer was extracted twice more with dichloromethane (about 50 mL each). T...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com