Mn<4+> doping red fluorescent material for white light LED and preparation method of Mn<4+> doping red fluorescent material
A technology of red fluorescence and white light, applied in luminescent materials, chemical instruments and methods, sustainable buildings, etc., can solve the problems of difficult control of the preparation process, unsuitable for large-scale production, unsuitable for industrial production, etc., to achieve good fluorescent thermal stability, Easy to promote operation, low cost effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0027] K 2 MnF 6Powder preparation:
[0028] At room temperature, 2.25g KMnO 4 Dissolve the powder in 150ml of 49wt% HF aqueous solution, stir evenly, add 45gKHF 2 The powder continued to stir for 40min, then cooled rapidly with ice water, and added 4ml of 49wt% H 2 o 2 Aqueous solution, when the color of the reactant solution changes from purple to brownish yellow, K 2 MnF 6 Suspension; let stand for 4h, centrifuge and wash (wash with acetone), and dry at 70°C for 6h to obtain K 2 MnF 6 Powder, spare.
Embodiment 2
[0030] Na 3 AlF 6 :0.5%Mn 4+ Preparation of fluorescent materials:
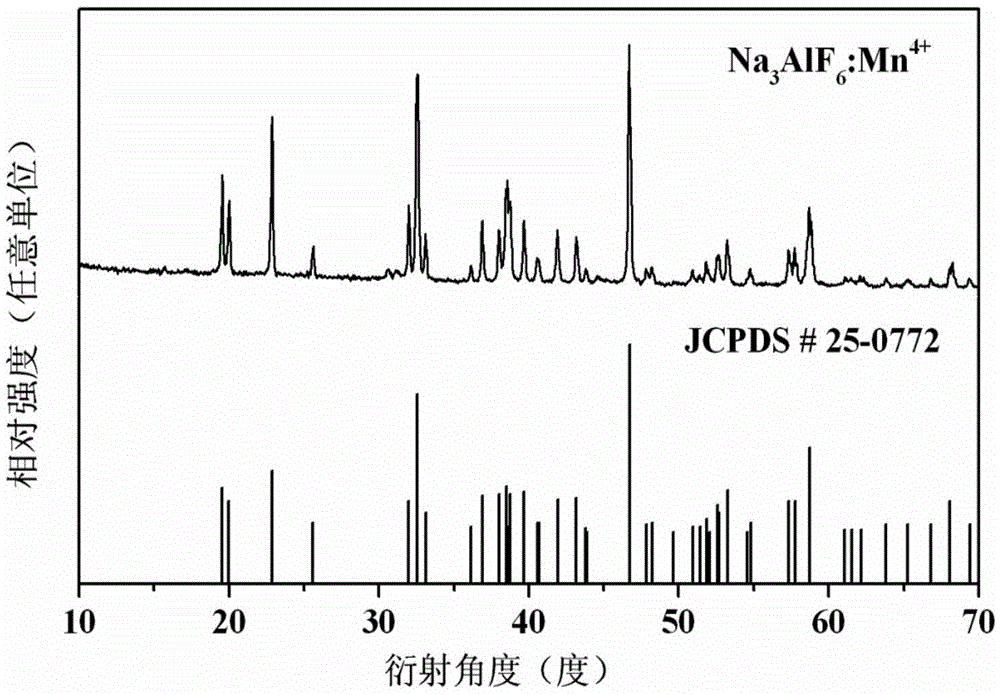
[0031] 0.00617gK 2 MnF 6 with 0.419gAlF 3 Dissolve in 10ml of hydrofluoric acid (49wt.%), stir for 10 minutes (stirring speed is 4000r / min), to obtain a transparent solution; then add 0.999g of NaOH powder to the transparent solution, continue stirring at room temperature for 30 minutes, and quickly cool to 4°C, centrifuge, wash with ethanol three times, and dry at 80°C for 7 hours to obtain Na 3 AlF 6 :0.5%Mn 4+ .
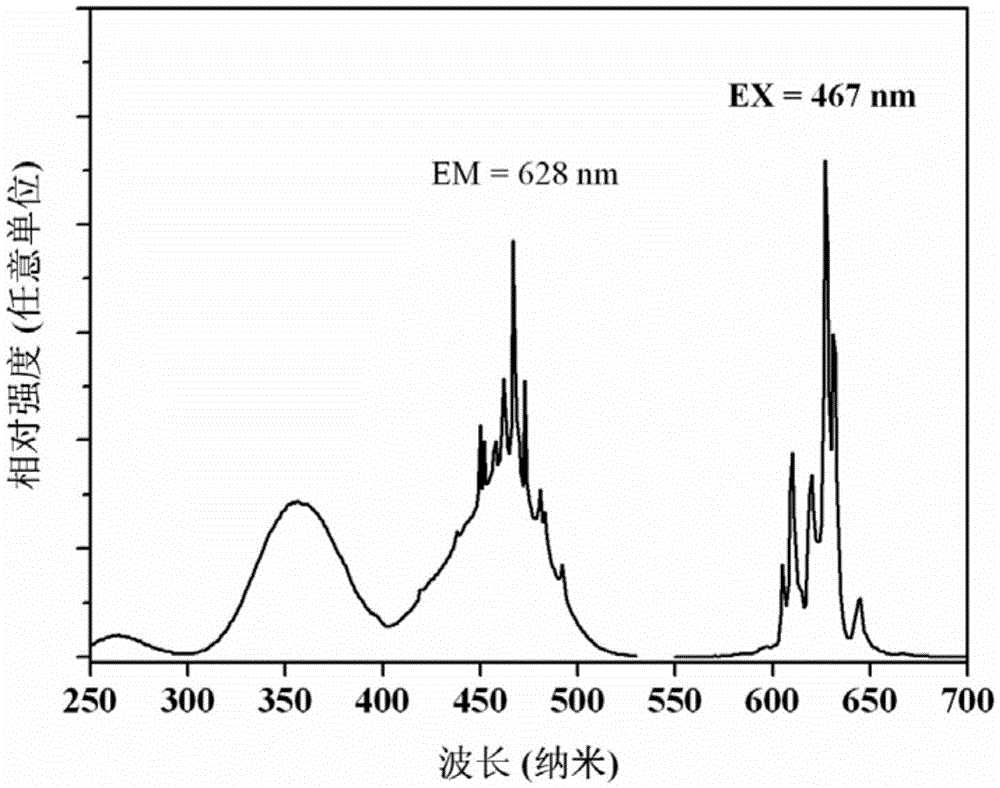
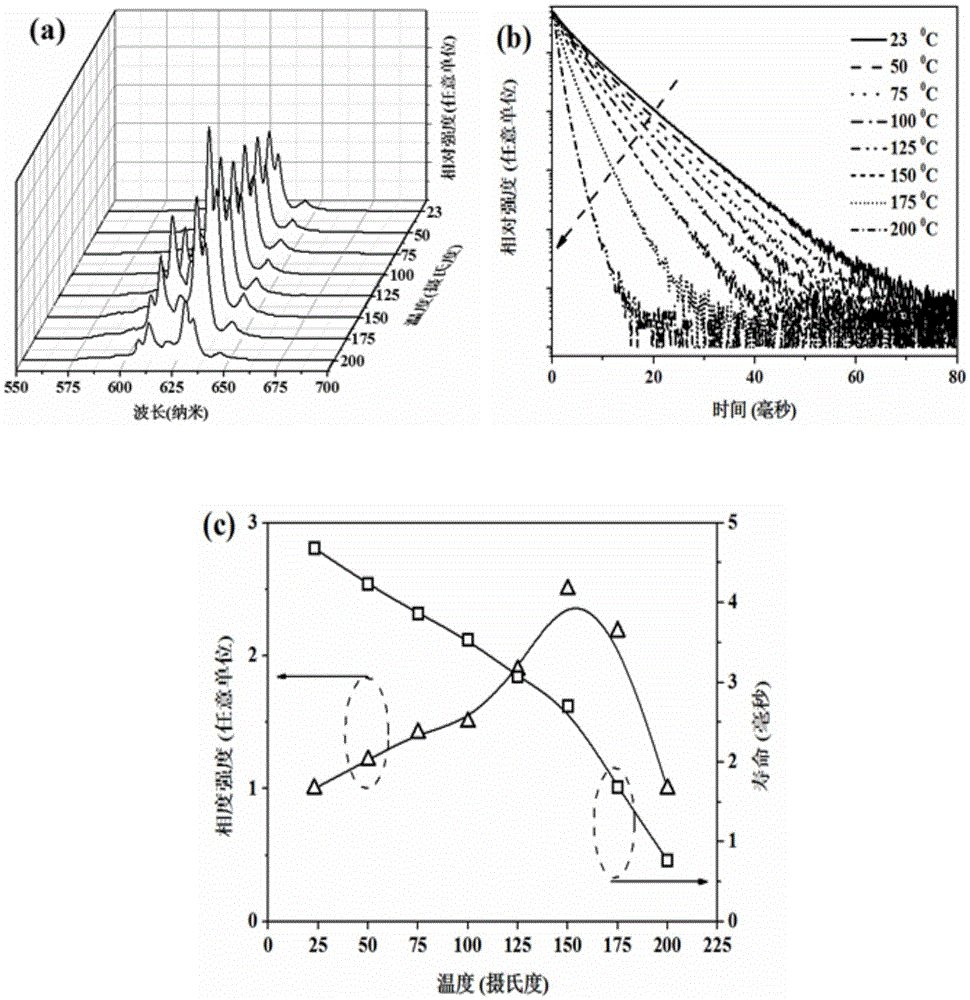
[0032] The sample glows bright red when excited by violet and blue light. The excitation spectrum of the sample consists of two broad bands at 357nm and 460nm, among which the strongest excitation band (460nm) perfectly matches the blue light emitted by the GaN blue chip, and the emission spectrum consists of six bands located at 606nm, 610nm, 620nm, 628nm, The sharp peaks at 632nm and 645nm are composed, and its color coordinates are located at: x=0.687, y=0.312, which belongs to pure ...
Embodiment 3
[0034] Na 3 AlF 6 :0.5%Mn 4+ Preparation of fluorescent materials:
[0035] 0.00617gK 2 MnF 6 with 0.419gAlF 3 Dissolved in 10ml of hydrofluoric acid (49wt.%), stirred for 10 minutes to obtain a transparent solution; then 2.649gNa 2 CO 3 Add the powder into the transparent solution, continue to stir at room temperature for 30 minutes (the stirring speed is 4000r / min), quickly cool to 4°C to obtain a light yellow precipitate, centrifuge, wash with acetone for 3 times, and dry at 80°C for 8h to obtain Na 3 AlF 6 :0.5%Mn 4+ .
[0036] The sample glows bright red when excited by violet and blue light. The excitation spectrum of the sample consists of two broad bands at 357nm and 460nm, among which the strongest excitation band (460nm) perfectly matches the blue light emitted by the GaN blue chip, and the emission spectrum consists of six bands located at 606nm, 610nm, 620nm, 628nm, The sharp peaks at 632nm and 645nm are composed, and its color coordinates are located a...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com