Method for carrying out catalytic synthesis on arene 2,2,2-trifluoro-ethyl sulfide through copper
A technology for synthesizing trifluoroethyl sulfide and aromatic hydrocarbons, which can be used in the preparation of sulfides, organic chemistry, etc., can solve the problems of inaccessibility and expensiveness of aromatic thiophenols, and achieves the effects of good adaptability, high yield, and simple reaction operation.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
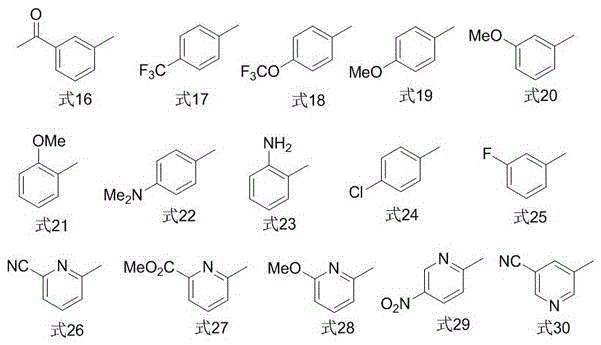
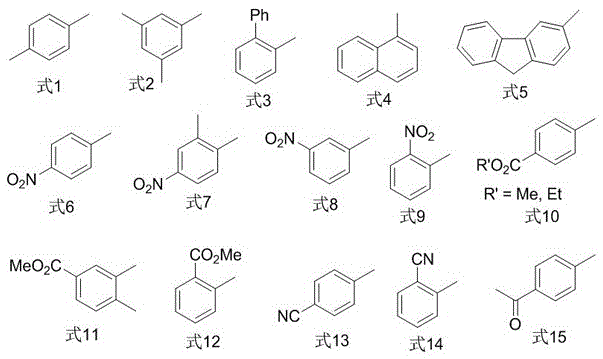
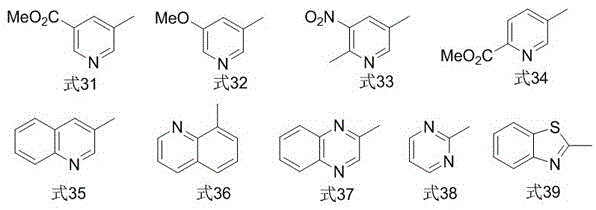
Image
Examples
Embodiment 1
[0020] In a nitrogen atmosphere, put a polytetrafluoroethylene magnet in a reactor, then add 0.050mmol cuprous iodide, 0.10mmol1,10-phenanthroline, 0.50mmol2-iodobenzoic acid methyl ester, 1.0mmol Sulfur powder, 1.0mmol 2,2,2-trifluoroiodoethane, 1.5mmol sodium borohydride, finally add 5mL N,N-dimethylformamide, stir and react in a closed system at 80°C for 16h, cool to room temperature, and use Diethyl ether was extracted 3 times, 10 mL each time, the organic phases were combined, washed 3 times with distilled water, the organic phase was dried with anhydrous magnesium sulfate, filtered, and then rotary evaporated to remove the organic solvent; Elution was carried out with alkane as the eluent to obtain methyl 2-(2,2,2-trifluoroethylthio)benzoate (isolated yield 83%). 1 HNMR (400MHz, CDCl 3 )δ7.96(d,J=8.6Hz,1H),7.59–7.42(m,2H),7.36–7.29(m,1H),3.96(s,3H),3.60(q,J=9.7Hz,2H ). 19 FNMR (376MHz, CDCl 3 )δ-65.2 (t, J=9.7Hz, 3F). GC-MSm / z250(M + ).
Embodiment 2
[0022] In a nitrogen atmosphere, put a polytetrafluoroethylene magnet in a reactor, then add 0.050mmol cuprous iodide, 0.10mmol1,10-phenanthroline, 0.50mmol2-cyanoiodobenzene, 1.0mmol sulfur powder, 1.0mmol 2,2,2-trifluoroiodoethane, 1.5mmol sodium borohydride, and finally add 5mL N,N-dimethylformamide, stir and react in a closed system at 80°C for 16h, cool to room temperature, and diethyl ether Extract 3 times, 10mL each time, combine the organic phases, wash 3 times with distilled water, dry the organic phases with anhydrous magnesium sulfate, filter, and then rotary evaporate to remove the organic solvent; Elution was performed as eluent to give 2-(2,2,2-trifluoroethylthio)benzonitrile (isolated yield 76%). 1 HNMR (400MHz, CDCl 3 )δ7.77–7.65(m,2H),7.60(t,J=7.7Hz,1H),7.45(t,J=7.8Hz,1H),3.58(q,J=9.5Hz,2H). 19 FNMR (376MHz, CDCl 3 )δ-66.1 (t, J=9.4Hz, 3F). GC-MSm / z217(M + ).
Embodiment 3
[0024] In a nitrogen atmosphere, put a polytetrafluoroethylene magnet in a reactor, then add 0.050mmol cuprous iodide, 0.10mmol1,10-phenanthroline, 0.50mmol1-iodonaphthalene, 1.0mmol sulfur powder, 1.0mmol 2,2,2-trifluoroiodoethane, 1.5mmol sodium borohydride, finally add 5mL N,N-dimethylformamide, stir and react in a closed system at 85°C for 16h, cool to room temperature, and extract with ether for 3 times, 10 mL each time, combined the organic phases, washed 3 times with distilled water, dried the organic phases with anhydrous magnesium sulfate, filtered, and then rotary evaporated to remove the organic solvent; The solvent was removed for elution to obtain 1-(2,2,2-trifluoroethyl)naphthalene sulfide (isolated yield 92%). 1 HNMR (400MHz, CDCl 3 )δ8.52(d,J=8.5Hz,1H),7.91(m,3H),7.63(m,2H),7.48(t,J=7.7Hz,1H),3.49(q,J=9.7Hz, 2H). 19 FNMR (376MHz, CDCl 3 )δ-66.0 (t, J=9.8Hz, 3F). GC-MSm / z242(M + ).
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com