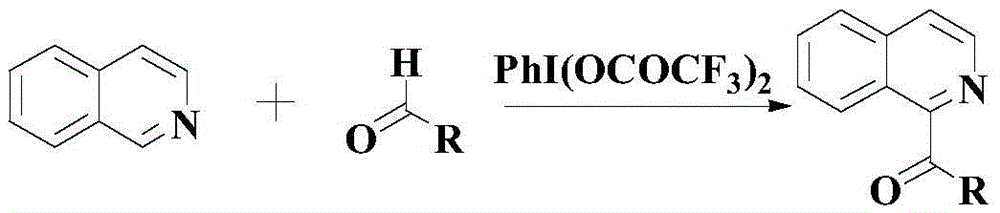
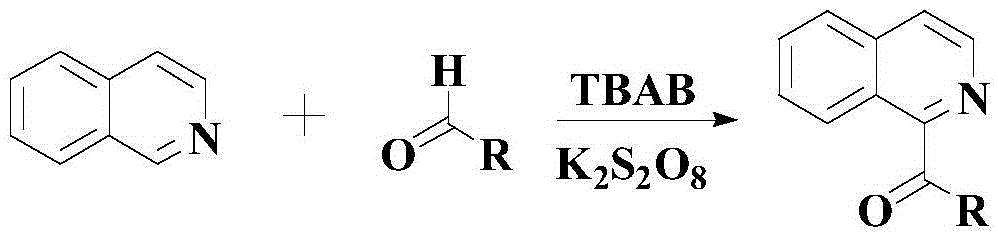
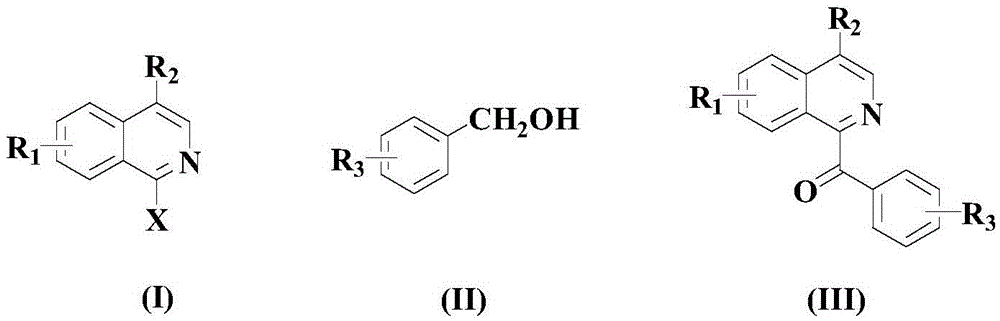
Synthetic method for drug intermediate diaryl ketone compound
A synthesis method and technology of diaryl ketones, applied in the direction of organic chemistry, etc., can solve the problems of low yield and achieve high yield and broad market application prospects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0035]
[0036] At room temperature, in a nitrogen atmosphere, add 100 mmol of the above formula (I) compound, 120 mmol of the above formula (II) compound, and 10 mmol of the catalyst NiCl to an appropriate amount of organic solvent ethylene glycol 2 (PCy 3 ) 2 , 150mmol oxidant cerium ammonium nitrate, 150mmol alkali DMPA and 10mmol accelerator (being the mixture of 2.5mmol cadmium acetate and 7.5mmol strontium nitrate), then warming up to 60°C, and stirring and reacting at this temperature for 10 hours;
[0037] After the reaction is over, filter the reaction liquid while it is hot, adjust the pH value of the filtrate to neutral, then fully wash with saturated brine, then add ethyl acetate to extract 2-3 times, combine the organic phases, dry over anhydrous sodium sulfate, reduce Concentrate under reduced pressure, and the resulting residue is subjected to silica gel column chromatography, eluting with a mixture of acetone and petroleum ether in an equal volume ratio, so...
Embodiment 2
[0040]
[0041] At room temperature, in a nitrogen atmosphere, to an appropriate amount of organic solvent ethylene glycol, add 100 mmol of the compound of the above formula (I), 150 mmol of the compound of the above formula (II), and 12 mmol of the catalyst NiCl 2 (PCy 3 ) 2 , 200mmol oxidant ceric ammonium nitrate, 175mmol alkali DMPA and 15mmol accelerator (being the mixture of 3mmol cadmium acetate and 12mmol strontium nitrate), then be warming up to 70 ℃, and at this temperature stirring reaction 8 hours;
[0042] After the reaction, the reaction solution was filtered while hot, the pH value of the filtrate was adjusted to neutral, then fully washed with saturated brine, and then extracted with ethyl acetate for 2-3 times, the organic phases were combined, dried over anhydrous sodium sulfate, reduced Concentrated under pressure, the obtained residue was subjected to silica gel column chromatography and rinsed with a mixture of acetone and petroleum ether in an equal vol...
Embodiment 3
[0045]
[0046] In a nitrogen atmosphere at room temperature, 100 mmol of the compound of the above formula (I), 180 mmol of the compound of the above formula (II), and 14 mmol of the catalyst NiCl were added to an appropriate amount of organic solvent ethylene glycol. 2 (PCy 3 ) 2 , 250mmol oxidant cerium ammonium nitrate, 200mmol alkali DMPA and 20mmol accelerator (being the mixture of 4.5mmol cadmium acetate and 15.5mmol strontium nitrate), then be warming up to 80 ℃, and at this temperature stirring reaction 6 hours;
[0047] After the reaction, the reaction solution was filtered while hot, the pH value of the filtrate was adjusted to neutral, then fully washed with saturated brine, and then extracted with ethyl acetate for 2-3 times, the organic phases were combined, dried over anhydrous sodium sulfate, reduced Concentrated under pressure, and the obtained residue was subjected to silica gel column chromatography and rinsed with a mixture of acetone and petroleum ethe...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com